OCR Specification focus:
‘Define enthalpy change of solution and enthalpy change of hydration for ionic substances in water.’
Introduction
Understanding enthalpy changes when ionic substances dissolve is essential for predicting solution behaviour. This subtopic focuses on defining enthalpy of solution and enthalpy of hydration, explaining their roles in ionic energetics.
Enthalpy Changes When Ionic Substances Dissolve
When an ionic compound dissolves in water, several energetic processes occur. These determine whether dissolution is exothermic or endothermic and influence solubility patterns across ionic substances.
Dissolving Ionic Lattices: An Overview
Breaking apart an ionic lattice and surrounding ions with water molecules involves two key enthalpy changes: enthalpy change of solution and enthalpy change of hydration. Each represents a distinct energetic step and must be precisely defined for OCR A-Level Chemistry.
Before exploring the energetics, it is essential to understand the formal terminology used to describe these steps.
Enthalpy Change of Solution: The enthalpy change when 1 mol of a solute dissolves in water to form a solution at standard conditions.
The dissolution process depends on the balance between forces breaking the ionic lattice and forces attracting ions to water molecules. These opposing effects create the overall enthalpy change of solution, which may be positive or negative depending on the substance.
Hydration of Gaseous Ions
After the lattice breaks apart, the separated gaseous ions interact strongly with polar water molecules. This interaction releases energy because water’s partial charges stabilise the ions.
Enthalpy Change of Hydration: The enthalpy change when 1 mol of gaseous ions becomes hydrated by water molecules, forming aqueous ions.
Hydration is usually strongly exothermic due to ion–dipole attractions, so it often offsets the energy needed to disrupt the ionic lattice. This is why some compounds dissolve readily whereas others do not.
When an ionic substance dissolves, water molecules surround the separated ions, forming hydration shells through ion–dipole attractions.
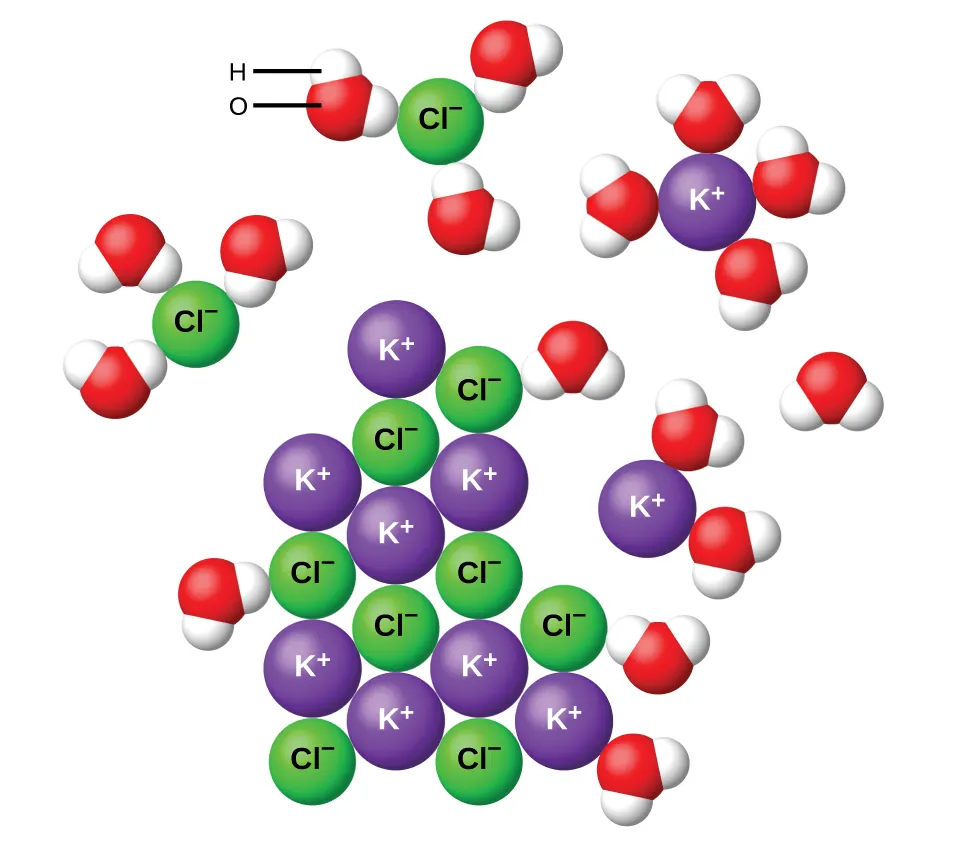
Hydration of K⁺ and Cl⁻ ions as potassium chloride dissolves in water. Water molecules orient so the oxygen end faces the cation and the hydrogen ends face the anion, showing stabilising ion–dipole attractions. Source
A full ionic dissolution can be viewed as the sum of these sequential steps, each with its characteristic energetic significance.
Why These Enthalpy Changes Matter
Understanding these enthalpy changes provides insight into solubility and helps predict whether dissolution is thermodynamically favourable. Ionic compounds do not dissolve simply because they dissociate; dissolution occurs only if the energetics allow it.
Breaking Down the Dissolution Process
The dissolution of an ionic solid can be conceptualised in three stages:
Disruption of the ionic lattice (endothermic; energy required).
Hydration of cations by surrounding water molecules (exothermic; energy released).
Hydration of anions through water–ion interactions (exothermic; energy released).
The overall enthalpy change of solution reflects the combination of these effects. A highly exothermic hydration step can compensate for a large amount of energy required to break the lattice.
The Role of Water’s Polarity
Water molecules possess a permanent dipole, with δ⁻ charge on oxygen and δ⁺ charges on hydrogen atoms. This polarity governs the hydration process because:
The δ⁻ oxygen end of water is attracted to cations.
The δ⁺ hydrogen ends are attracted to anions.
These interactions stabilise the ions, which is why hydration enthalpies are always negative.
Factors Affecting Hydration Enthalpy
Even though this subsubtopic focuses on definitions, it is important to recognise that not all ions hydrate in the same way. Variations in enthalpy of hydration arise from structural and electrostatic differences among ions.
Ionic Radius
Smaller ions produce stronger ion–dipole attractions because water molecules can approach their centres more closely. This leads to:
More negative (exothermic) hydration enthalpies
Greater stabilisation in aqueous solution
Ionic Charge
Higher-charge ions attract water molecules more strongly. This results in:
Larger hydration enthalpies
Highly favourable hydration steps that significantly influence dissolution energetics
Both factors determine how much hydration compensates for the lattice enthalpy required to break the solid structure.
Relationship Between Solution and Hydration Enthalpies
Because dissolution involves both breaking the lattice and hydrating the resulting ions, the enthalpy of solution links directly to hydration enthalpies.
Conceptual Connection
The overall enthalpy change of solution can be expressed as:
Energy to break the lattice (endothermic)
Plus energy released when ions are hydrated (exothermic)
These interactions determine whether a substance dissolves readily, dissolves slowly, or does not dissolve significantly at all.
The enthalpy change of hydration is always exothermic because new attractions form between the ion and surrounding water molecules.
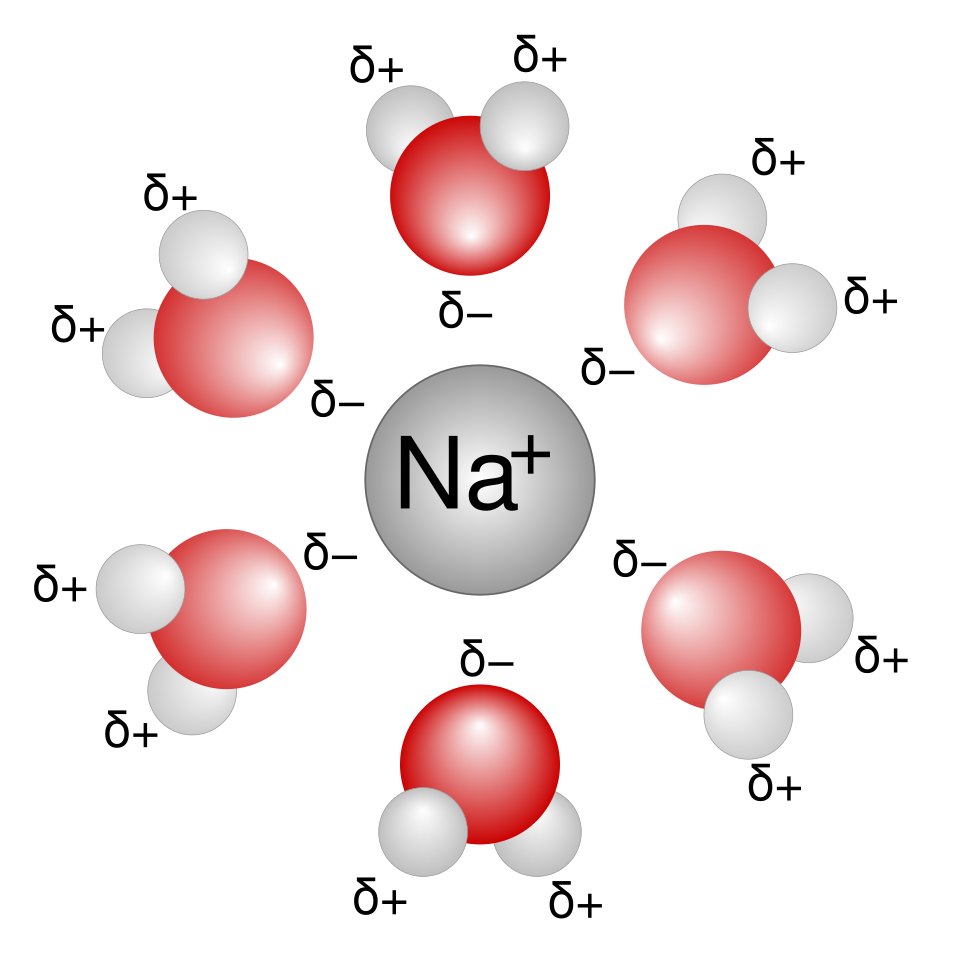
A simplified hydration shell around a sodium ion, showing the oxygen (δ−) ends of water molecules directed towards Na⁺. This illustrates ion–dipole attractions formed during hydration in aqueous solution. Source
Enthalpy of Solution (ΔsolH) = Σ Hydration Enthalpies (ΔhydH) − Lattice Enthalpy (ΔLEH)
ΔsolH = Enthalpy change of solution, kJ mol⁻¹
ΔhydH = Enthalpy change of hydration for each ion, kJ mol⁻¹
ΔLEH = Lattice enthalpy, kJ mol⁻¹
Understanding this relationship makes it clear why dissolving cycles feature lattice and hydration enthalpies side-by-side. If hydration releases more energy than breaking the lattice requires, dissolution becomes energetically favourable.
A positive or negative enthalpy of solution reflects the balance of these contributions rather than a single dominant process, reinforcing why precise definitions are essential in this topic.
FAQ
Hydration is always exothermic because energy is released when attractive forces form between ions and polar water molecules.
Ion–dipole attractions are stronger than the forces between water molecules alone, so forming hydration shells lowers the overall energy of the system. As new interactions are formed rather than broken, the enthalpy change is negative for all ions.
A positive enthalpy change of solution means the process is endothermic, but dissolution can still occur if other factors favour it.
These include:
An increase in entropy when a solid lattice becomes dispersed ions
Strong hydration of ions stabilising them in solution
Thermodynamic feasibility depends on both enthalpy and entropy, not enthalpy alone.
Using gaseous ions allows hydration enthalpy to represent only the interaction between ions and water.
If solid ions were used, the value would also include lattice enthalpy, making it impossible to isolate hydration effects. Defining hydration from gaseous ions ensures values are comparable and can be used reliably in energy cycles.
Hydration enthalpy shows how strongly ions are stabilised in water.
Compounds with ions that have:
High charge
Small ionic radius
tend to have more exothermic hydration enthalpies, which can offset large lattice enthalpies and promote solubility. Weak hydration may lead to poor solubility even if the lattice is not especially strong.
Hydration enthalpy cannot be measured directly because gaseous ions cannot be isolated under normal laboratory conditions.
Instead, values are obtained indirectly using enthalpy cycles that combine measurable quantities such as lattice enthalpy and enthalpy of solution. This indirect approach allows accurate determination while remaining experimentally feasible.
Practice Questions
Define the enthalpy change of hydration and state whether it is endothermic or exothermic.
(2 marks)
Correct definition: enthalpy change when 1 mol of gaseous ions becomes hydrated by water molecules (1 mark)
Correct statement that the enthalpy change of hydration is exothermic / negative (1 mark)
An ionic solid dissolves in water to form an aqueous solution.
a) Define the enthalpy change of solution. (2 marks)
b) Explain, in terms of energetic changes, why the enthalpy change of solution for an ionic compound can be either positive or negative. (3 marks)
(5 marks)
a)
Enthalpy change when 1 mol of a solute dissolves in water (1 mark)
To form a solution at standard conditions / infinite dilution implied (1 mark)
b)
Energy is required to break the ionic lattice (endothermic process) (1 mark)
Energy is released when gaseous ions are hydrated by water molecules (exothermic process) (1 mark)
Overall enthalpy change depends on the balance between lattice enthalpy and hydration enthalpies, so it may be positive or negative (1 mark)

