OCR Specification focus:
‘Qualitatively explain how ionic charge and radius affect lattice enthalpy and enthalpy of hydration.’
Lattice and hydration enthalpies explain trends in ionic compound stability and solubility by relating ionic charge and ionic radius to electrostatic attractions.
Understanding Enthalpy Trends in Ionic Systems
Trends in lattice enthalpy and enthalpy of hydration arise from electrostatic forces between charged particles. These trends are central to understanding ionic bonding strength, solubility, and the energetic feasibility of dissolving ionic compounds in water.
Both enthalpy changes depend primarily on:
The charge on the ions
The radius (size) of the ions
Although lattice enthalpy and hydration enthalpy describe different processes, the same fundamental electrostatic principles govern both.
Lattice Enthalpy and Ionic Properties
Lattice enthalpy describes the energy change when gaseous ions form an ionic lattice. It reflects the strength of ionic bonding within a solid crystal.
Lattice enthalpy: The enthalpy change when one mole of an ionic solid is formed from its gaseous ions under standard conditions.
Stronger ionic attractions release more energy, resulting in a more negative lattice enthalpy.
Effect of Ionic Charge on Lattice Enthalpy
Increasing ionic charge significantly increases lattice enthalpy because electrostatic attraction is proportional to charge magnitude.
Key qualitative trends:
Ions with higher charges form stronger electrostatic attractions
Stronger attractions release more energy when the lattice forms
This produces higher magnitude (more negative) lattice enthalpy values
For example:
Compounds containing 2+ or 3+ cations typically have much larger lattice enthalpies than those containing 1+ cations
The same trend applies to higher charged anions
The effect of charge is often more influential than size when comparing lattice enthalpies.
Effect of Ionic Radius on Lattice Enthalpy
Ionic radius affects the distance between oppositely charged ions in the lattice.
Qualitative effects of radius:
Smaller ions allow ions to pack more closely
Shorter interionic distances increase electrostatic attraction
This leads to stronger bonding and a more negative lattice enthalpy
Trends down a group:
Ionic radius increases
Distance between ions increases
Lattice enthalpy decreases in magnitude
For ions with the same charge, radius is the dominant factor determining lattice enthalpy.
This graph shows how lattice energy decreases as ionic radius increases, both for larger halide ions and larger alkali metal cations. It visually reinforces that increased interionic distance weakens electrostatic attraction in ionic lattices. Quantitative values are shown for illustration, although OCR requires only qualitative understanding. Source
Enthalpy of Hydration and Ionic Properties
Enthalpy of hydration refers to the interaction between ions and water molecules when an ionic compound dissolves.
Enthalpy of hydration: The enthalpy change when one mole of gaseous ions is dissolved in water to form aqueous ions under standard conditions.
This process involves ion–dipole attractions between ions and polar water molecules.
Effect of Ionic Charge on Enthalpy of Hydration
Higher ionic charge increases the strength of attraction between ions and water molecules.
Key qualitative trends:
Higher charged ions attract water molecules more strongly
More energy is released during hydration
Enthalpy of hydration becomes more negative
This applies to both cations and anions. For example:
A 2+ cation will typically have a more negative hydration enthalpy than a 1+ cation of similar size
Effect of Ionic Radius on Enthalpy of Hydration
Ionic radius strongly influences hydration because it affects charge density.
Charge density depends on:
Ionic charge
Ionic radius
Smaller ions have higher charge density, resulting in stronger attractions to water molecules.
Qualitative trends:
Smaller ions → higher charge density
Stronger ion–dipole attractions
More energy released during hydration
More negative enthalpy of hydration
As ionic radius increases down a group:
Charge density decreases
Enthalpy of hydration becomes less negative
In hydration, water molecules orient so the δ− oxygen faces cations, giving strong ion–dipole attractions.
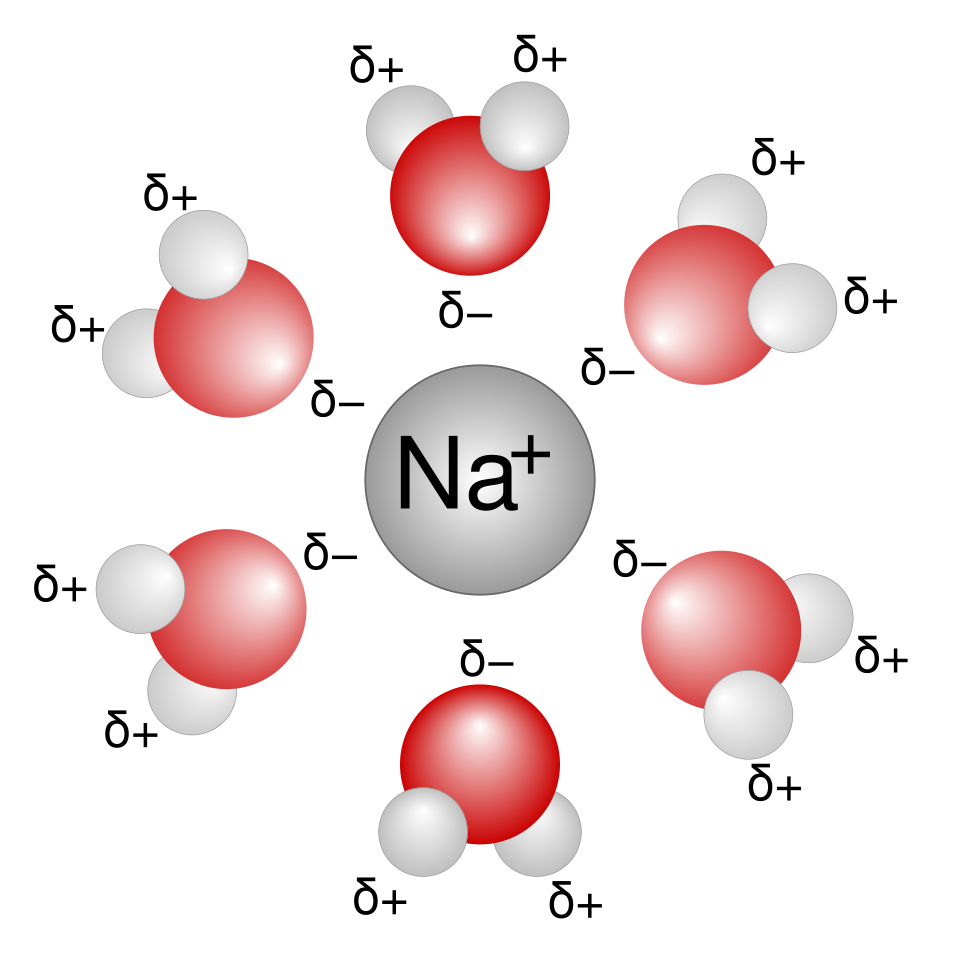
This diagram illustrates hydration of a sodium ion by polar water molecules, highlighting ion–dipole attraction. The orientation of water reflects how charge density influences hydration enthalpy. Only a single ion is shown, but the same principle applies when comparing ions of different sizes and charges. Source
Comparing Lattice Enthalpy and Hydration Enthalpy Trends
Although both enthalpy changes depend on charge and radius, they describe opposing processes.
Key contrasts:
Lattice enthalpy favours strong ion–ion attractions in the solid state
Hydration enthalpy favours strong ion–water attractions in solution
Shared qualitative trends:
Higher ionic charge increases both lattice enthalpy magnitude and hydration enthalpy magnitude
Smaller ionic radius increases both lattice enthalpy magnitude and hydration enthalpy magnitude
However, the relative sizes of these enthalpy changes differ between compounds, influencing solubility and solution energetics.
Importance of Charge Density
Charge density provides a unifying explanation for both trends.
High charge density results from:
High ionic charge
Small ionic radius
Consequences of high charge density:
Strong electrostatic attractions in ionic lattices
Strong ion–dipole attractions with water
Large magnitude lattice and hydration enthalpies
Ions with very high charge density can form extremely stable lattices, sometimes making hydration insufficient to overcome lattice enthalpy during dissolution.
Qualitative Nature of OCR Requirements
The OCR specification requires qualitative explanations, not numerical comparisons.
Students should be able to:
Explain trends using ideas of charge, radius, and electrostatic attraction
Compare ions and compounds without calculations
Link ionic properties to energetic strength and stability
Clear use of correct terminology, including lattice enthalpy, enthalpy of hydration, ionic charge, ionic radius, and charge density, is essential for accurate explanations.
FAQ
Electrostatic attraction depends directly on the magnitude of charge. Doubling the charge increases attraction far more than a similar proportional change in distance.
As a result, differences in ionic charge often dominate lattice enthalpy trends, even when ionic radii are relatively similar.
This is why compounds containing 2+ or 3+ ions often have much larger lattice enthalpies than those with only 1+ and 1− ions.
Charge density combines the effects of charge and radius into a single idea.
High charge density occurs when an ion:
Has a high ionic charge
Has a small ionic radius
High charge density strengthens electrostatic attractions in ionic lattices and ion–dipole attractions with water, explaining why both lattice enthalpy and hydration enthalpy increase in magnitude under these conditions.
Small, highly charged ions produce a strong electric field.
This electric field pulls the electron density in nearby water molecules towards the ion, strengthening ion–dipole attraction.
Stronger polarisation leads to more energy being released during hydration, making the enthalpy of hydration more negative.
As you move down a group, ionic radius increases while charge remains constant.
The increased size spreads the charge over a larger volume, reducing charge density.
Lower charge density weakens ion–dipole attraction with water molecules, so less energy is released during hydration and the enthalpy of hydration becomes less negative.
Very high lattice enthalpy indicates extremely strong ion–ion attractions in the solid.
If the energy released during hydration is not sufficient to overcome this lattice energy, dissolution is energetically unfavourable.
This explains why some ionic compounds with small, highly charged ions are sparingly soluble or insoluble in water.
Practice Questions
Magnesium oxide has a much more negative lattice enthalpy than sodium chloride.
Explain this difference in terms of ionic properties.
(2 marks)
Award one mark for each correct point:
Magnesium oxide contains ions with higher charges (Mg²⁺ and O²⁻) than sodium chloride (Na⁺ and Cl⁻), leading to stronger electrostatic attraction. (1 mark)
Stronger electrostatic attraction between oppositely charged ions results in a more negative lattice enthalpy for magnesium oxide. (1 mark)
The enthalpy of hydration of the aluminium ion, Al³⁺, is much more negative than that of the sodium ion, Na⁺.
Explain, using ideas of ionic charge, ionic radius, and charge density, why the enthalpy of hydration of Al³⁺ is more negative than that of Na⁺.
(5 marks)
Marks may be awarded as follows:
Al³⁺ has a higher ionic charge than Na⁺. (1 mark)
Al³⁺ has a smaller ionic radius than Na⁺. (1 mark)
Higher charge and smaller radius give Al³⁺ a higher charge density. (1 mark)
Higher charge density causes stronger ion–dipole attractions between Al³⁺ and water molecules. (1 mark)
Stronger ion–dipole attractions release more energy, making the enthalpy of hydration of Al³⁺ more negative than that of Na⁺. (1 mark)
Allow alternative correct wording. Do not award marks for calculations or numerical values.

