OCR Specification focus:
‘Define lattice enthalpy (formation of 1 mol lattice from gaseous ions) as a measure of ionic bond strength.’
Lattice enthalpy underpins understanding of ionic bonding by quantifying electrostatic attractions, allowing chemists to compare ionic compounds and explain trends in stability and reactivity.
Understanding Ionic Bonding Energetics
Ionic bonding arises from electrostatic attraction between oppositely charged ions arranged in a giant ionic lattice. While ionic bonds are often described qualitatively as “strong” or “weak”, thermodynamics provides a quantitative measure of this strength. This is achieved through the concept of lattice enthalpy, which reflects how much energy is associated with forming or separating an ionic lattice.
In OCR A-Level Chemistry, lattice enthalpy is treated as a fundamental energetic quantity that links ionic charge, ionic radius, and bond strength. It is essential for explaining why some ionic compounds are more stable than others and why certain salts have high melting points.
Defining Lattice Enthalpy
The term lattice enthalpy has a precise meaning and must be used carefully, particularly regarding sign conventions and physical states.
Lattice enthalpy: The enthalpy change when one mole of an ionic solid lattice is formed from its gaseous ions under standard conditions.
This diagram illustrates lattice formation for sodium chloride by bringing Na⁺(g) and Cl⁻(g) together to form NaCl(s), highlighting the enthalpy change called lattice enthalpy. It reinforces that lattice enthalpy is a measure of ionic bonding strength because it reflects the attraction between oppositely charged ions in a lattice. Source
This definition emphasises several key points:
The process involves gaseous ions, not aqueous ions or solid elements.
Exactly one mole of the ionic lattice is formed.
The enthalpy change refers specifically to lattice formation, not lattice dissociation.
Because forming an ionic lattice involves strong attractions between ions, lattice enthalpy of formation is always exothermic. Energy is released as ions come together, so lattice enthalpy values are negative when defined in this way.
It is important to recognise that some textbooks define lattice enthalpy as lattice dissociation enthalpy instead. OCR uses the formation definition, so careful attention to wording and sign is essential.
Lattice Enthalpy as a Measure of Ionic Bond Strength
Lattice enthalpy provides a direct measure of ionic bond strength. The more negative the lattice enthalpy, the stronger the ionic bonding within the lattice.
Stronger ionic bonding means:
Greater electrostatic attraction between ions
Higher stability of the ionic solid
Higher melting and boiling points
This relationship arises because lattice enthalpy reflects the total energy released when all ions in the lattice interact. Large energy release indicates very strong attractions operating throughout the crystal structure.
When comparing ionic compounds, lattice enthalpy allows meaningful comparisons beyond simple formulas. For example, compounds containing small, highly charged ions typically have much more negative lattice enthalpies than those containing larger, singly charged ions.
Factors Affecting Ionic Bond Strength
Although detailed trends are covered elsewhere, understanding lattice enthalpy requires awareness of the underlying electrostatic principles.
Ionic bond strength depends on:
Magnitude of ionic charge
Distance between ion centres (ionic radius)
These factors align with Coulomb’s law, which describes electrostatic attraction. Higher charges increase attraction, while larger ionic radii increase the distance between charges, reducing attraction.
For example:
Ions with charges of 2+ and 2− attract each other more strongly than ions with charges of 1+ and 1−.
Smaller ions allow closer approach, increasing attraction and energy release.
Thus, lattice enthalpy captures how effectively ions attract each other within a lattice.
Introducing Ionic Strength
To discuss lattice enthalpy meaningfully, it is helpful to consider ionic strength, which relates to how strongly ions interact due to their charge.
Ionic strength: A qualitative measure of the intensity of electrostatic interactions between ions, influenced primarily by ionic charge and ionic radius.
In the context of lattice enthalpy, ionic strength does not refer to a calculated solution property but rather to the strength of ionic interactions within a solid lattice. High ionic strength corresponds to strong attractions between ions.
Ionic strength increases when:
Ionic charge increases
Ionic radius decreases
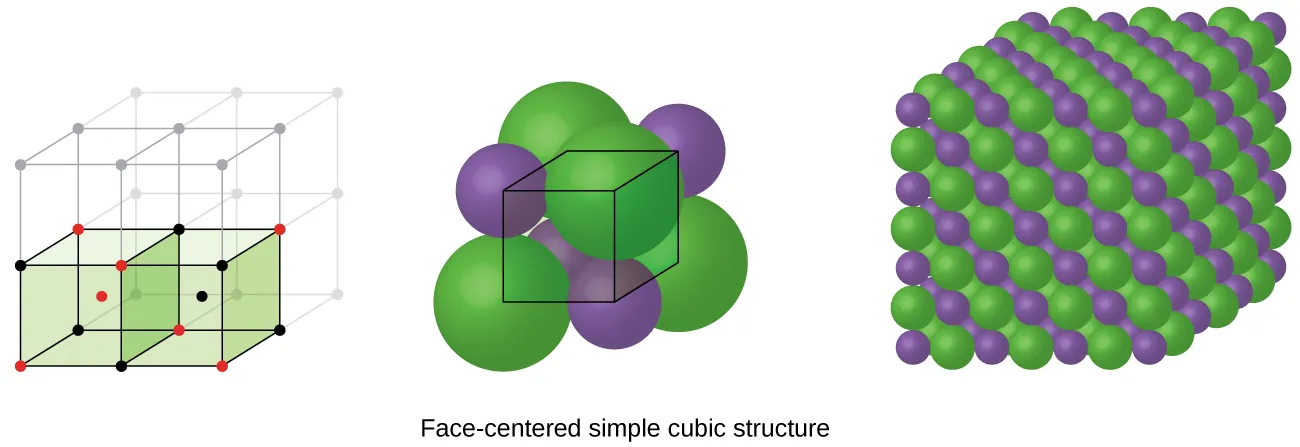
This figure shows how ions pack into a repeating crystal lattice and uses sodium chloride to illustrate how a large anion and smaller cation fit into the structure. The emphasis on ionic radii helps connect ion size to the strength of electrostatic attraction in the lattice. Extra detail included: unit cells and octahedral holes are shown, which go beyond the syllabus requirement. Source
This makes ionic strength a useful conceptual tool for explaining differences in lattice enthalpy between ionic compounds.
Linking Ionic Strength and Lattice Enthalpy
Lattice enthalpy can be understood as the energetic consequence of ionic strength across an entire crystal lattice. Each ion experiences attractions to many neighbouring ions, and the cumulative effect determines the lattice enthalpy value.
Strong ionic strength leads to:
Very negative lattice enthalpy values
Tightly bound ionic lattices
High resistance to separation into individual ions
Weaker ionic strength results in:
Less negative lattice enthalpy values
More easily disrupted lattices
Lower thermal stability
By framing lattice enthalpy in terms of ionic strength, students can clearly see how microscopic properties of ions translate into macroscopic properties of solids.
Importance in OCR A-Level Chemistry
Lattice enthalpy plays a central role in thermochemical analysis of ionic compounds. It provides:
A quantitative measure of ionic bond strength
A foundation for understanding energetic cycles involving ionic solids
A conceptual bridge between structure, bonding, and energy
Mastering the precise definition and interpretation of lattice enthalpy is essential for later topics involving enthalpy cycles, stability comparisons, and the energetics of ionic processes, all of which rely on this core concept.
FAQ
Using gaseous ions ensures that lattice enthalpy measures only the energy change due to ionic attractions.
If solid or aqueous ions were used, additional energy changes such as atomisation or hydration would be involved, making comparisons between compounds unreliable.
Defining lattice enthalpy from gaseous ions provides a consistent reference state for all ionic solids.
Lattice formation involves oppositely charged ions moving closer together.
As electrostatic attraction increases:
Potential energy decreases
Energy is released to the surroundings
This energy release makes the enthalpy change negative, reflecting the stability gained when the ionic lattice forms.
A more negative lattice enthalpy indicates stronger ionic attractions within the lattice.
Stronger attractions require more energy to overcome, so:
Ionic solids with very negative lattice enthalpies have high melting points
Those with weaker attractions melt at lower temperatures
This relationship explains why compounds like magnesium oxide melt at much higher temperatures than sodium chloride.
Lattice enthalpy depends on both charge and size of ions.
Comparing values without noting:
Ionic charge
Ionic radius
can lead to incorrect conclusions. For example, a compound with a less negative lattice enthalpy may still be stable if it contains large ions with lower charge density.
Lattice enthalpy applies specifically to giant ionic lattices formed by electrostatic attraction.
Covalent substances do not form ionic lattices and therefore have no lattice enthalpy value.
This makes lattice enthalpy a useful indicator for identifying strongly ionic compounds and distinguishing them from covalent or molecular solids.
Practice Questions
Define lattice enthalpy as used in OCR A-Level Chemistry and explain what information it provides about an ionic compound.
(2 marks)
Award marks as follows:
1 mark for a correct definition: lattice enthalpy is the enthalpy change when one mole of an ionic lattice is formed from gaseous ions.
1 mark for stating that lattice enthalpy is a measure of ionic bond strength or strength of electrostatic attraction in the lattice.
Explain why magnesium oxide has a more negative lattice enthalpy than sodium chloride.
Your answer should refer to ionic charge, ionic radius, and electrostatic attraction.
(5 marks)
Award marks as follows:
1 mark for stating that magnesium oxide contains Mg2+ and O2− ions, whereas sodium chloride contains Na+ and Cl− ions.
1 mark for explaining that higher ionic charges lead to stronger electrostatic attraction.
1 mark for stating that Mg2+ and O2− ions have smaller ionic radii than Na+ and Cl− ions.
1 mark for explaining that smaller ionic radius results in a shorter distance between ion centres.
1 mark for linking stronger electrostatic attraction to a more negative lattice enthalpy.
Maximum 5 marks.

