OCR Specification focus:
‘Compare entropies of solids, liquids and gases; relate changes to numbers of gaseous molecules.’
Entropy compares the extent of energy dispersal between physical states and reactions, helping predict entropy changes by examining states of matter and gaseous particle numbers.
Understanding Entropy in Different States
Entropy and Physical State
Entropy is a measure of how dispersed energy is within a system and how many possible arrangements particles can adopt. Different physical states exhibit characteristic entropy values due to differences in particle movement and spacing.
Entropy: A measure of the dispersal of energy and the degree of disorder in a system.
A solid, liquid, and gas containing the same substance will not have equal entropy values. This is because the particles behave very differently in each state, leading to differences in available energy arrangements.
Entropy of Solids
Solids generally have the lowest entropy of the three physical states. This is because:
Particles are arranged in a fixed, regular lattice
Particles vibrate about fixed positions only
There are relatively few possible arrangements of energy
The restricted motion means energy is poorly dispersed, resulting in low entropy. Crystalline solids in particular have very low entropy due to their highly ordered structures.
Entropy of Liquids
Liquids have higher entropy than solids but lower than gases. In liquids:
Particles are close together but not fixed in place
Particles can move past one another
There are more possible arrangements than in solids
The increased freedom of movement allows energy to be spread among more arrangements, increasing entropy compared to solids. However, limited spacing still restricts dispersal relative to gases.
Entropy of Gases
Gases possess the highest entropy of the three states. This is because:
Particles are far apart
Particles move rapidly and randomly
There is a very large number of possible energy arrangements
The wide spacing and high mobility allow energy to be dispersed throughout a much larger volume. This extensive dispersal results in a high entropy value.
Comparing Entropies of States
General Trend
For a given substance under similar conditions, entropy follows the trend:
Solid < Liquid < Gas
For the same substance at the same temperature, S(g) > S(l) > S(s) because particles become progressively freer to move.
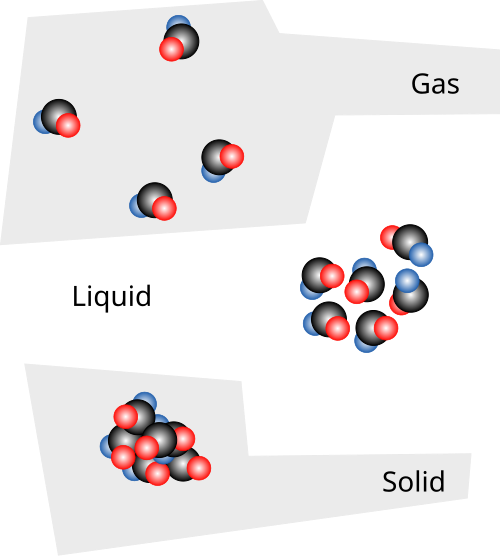
This diagram compares particle arrangements in the solid, liquid, and gaseous states. Solids show closely packed, ordered particles; liquids are close but disordered; gases are widely spaced with much greater freedom of motion, consistent with higher entropy. Source
This trend is fundamental for OCR A-Level Chemistry and is frequently applied when predicting the direction of entropy change during physical and chemical processes.
Changes of State and Entropy
When a substance changes state, its entropy changes accordingly:
Melting (solid → liquid) increases entropy
Boiling (liquid → gas) increases entropy significantly
Freezing or condensation decrease entropy
At phase changes, entropy increases sharply, with a much larger increase on vaporisation than on fusion.
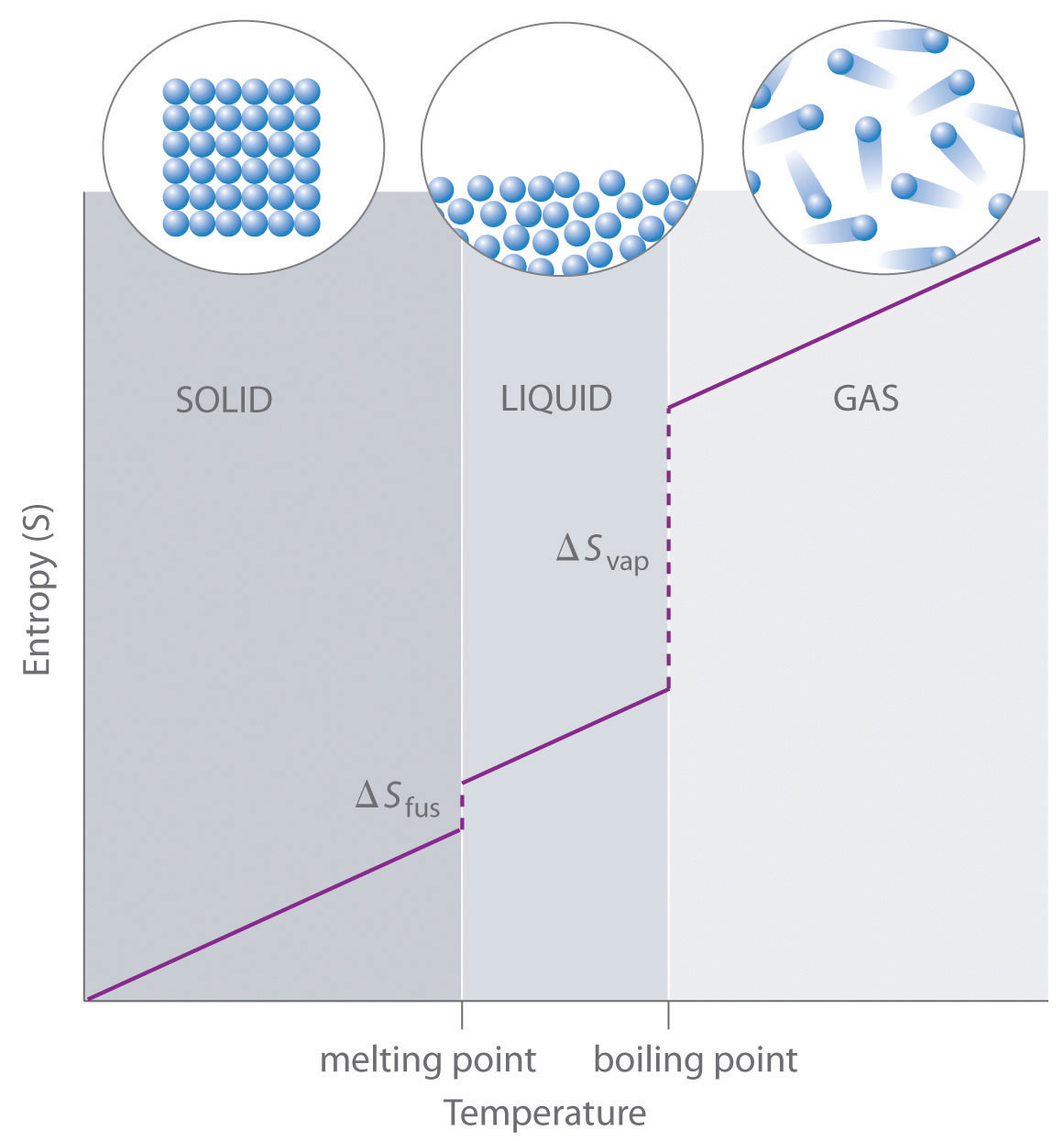
The graph shows entropy rising with temperature, with pronounced jumps at the melting and boiling points. The step increases reflect the much greater dispersal of energy when particles gain freedom of motion during changes of state. Source
The largest entropy change usually occurs during vaporisation, reflecting the major increase in particle separation and freedom.
Entropy and Gaseous Molecules in Reactions
Importance of Gaseous Particle Numbers
In chemical reactions, entropy changes are strongly influenced by changes in the number of gaseous molecules. Gases dominate entropy considerations because they already have high entropy values.
When comparing reactants and products:
An increase in the number of moles of gas usually results in a positive entropy change
A decrease in the number of moles of gas usually results in a negative entropy change
This relationship allows qualitative predictions of entropy change without numerical calculation.
Examples of Gaseous Changes
Consider reactions involving gases:
Reactions producing more gas particles increase disorder
Reactions consuming gas particles reduce disorder
For example:
One mole of gas forming two moles of gas increases entropy
Two moles of gas forming one mole of gas decreases entropy
The key factor is not the identity of the gas, but the number of gaseous particles present.
Using Standard Entropy Values
Standard Entropy
Entropy values used in calculations are measured under standard conditions and are known as standard entropy values.
Standard entropy (S°): The entropy of one mole of a substance under standard conditions, measured in J K⁻¹ mol⁻¹.
These values reflect both physical state and molecular complexity. For example:
Gases have much higher S° values than liquids or solids
Larger, more complex molecules tend to have higher entropy
Comparing Reactions Using Entropy
To compare entropy changes in reactions, chemists often calculate the overall entropy change using standard entropy values.
Entropy change of reaction (ΔS) = ΣS°(products) − ΣS°(reactants)
ΔS = Entropy change of reaction (J K⁻¹ mol⁻¹)
S° = Standard entropy value (J K⁻¹ mol⁻¹)
Although calculations are covered elsewhere, the qualitative interpretation is essential here. A positive ΔS indicates greater energy dispersal in products, often associated with increased gaseous particle numbers or changes to more disordered states.
Gases have much higher entropy because particles move freely in three dimensions, giving many more possible arrangements (microstates).
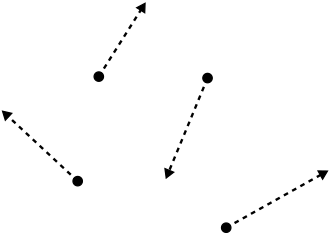
This schematic illustrates random translational motion of gas particles. The high freedom of movement allows many possible arrangements of energy, explaining why gases have much higher entropy than solids or liquids. Source
Qualitative Predictions of Entropy Change
When asked to compare or predict entropy changes, OCR expects students to:
Identify physical states involved
Count gaseous molecules on each side of the equation
Consider changes in particle freedom and dispersal
Key qualitative rules include:
Formation of gases increases entropy
Reduction in gas particles decreases entropy
Solid or liquid forming gas greatly increases entropy
These principles allow reliable comparisons without numerical data and are essential for understanding feasibility and free energy later in the course.
FAQ
Gases have far greater particle separation than liquids, allowing particles to occupy many more positions in space.
This leads to a much larger number of possible energy arrangements, known as microstates, which directly increases entropy.
Although liquids can flow, their particles remain relatively close together, limiting energy dispersal compared with gases.
More complex gas molecules usually have higher entropy than simpler ones.
This is because:
They have more atoms that can move independently
Energy can be distributed among more vibrational and rotational modes
As a result, larger gaseous molecules have more ways to store and disperse energy.
Gases already possess very high entropy, so changes involving gases have a much greater impact on overall entropy change.
Small changes in the number of gaseous particles can outweigh much larger changes involving solids or liquids, whose entropy values are relatively low and similar.
Yes, entropy increases with temperature for all states because particles gain kinetic energy.
However, even at higher temperatures:
Solids still have lower entropy than liquids
Liquids still have lower entropy than gases
The relative order of entropy between states remains the same under comparable conditions.
Forming a gas introduces a dramatic increase in particle freedom and volume occupied.
Particles move from a restricted environment to one where they can spread throughout the container, creating a large increase in possible energy arrangements and energy dispersal.
Practice Questions
A substance can exist as a solid, liquid, or gas.
State and explain how the entropy of the substance changes when it changes from a liquid to a gas.
(2 marks)
Question 1 (2 marks)
States that entropy increases when a liquid changes to a gas. (1 mark)
Explains that gas particles are further apart and move more freely, giving greater energy dispersal or more possible arrangements. (1 mark)
Consider the following two reactions, all substances being in the gaseous state unless stated otherwise.
Reaction A:
N2(g) + 3H2(g) → 2NH3(g)
Reaction B:
CaCO3(s) → CaO(s) + CO2(g)
For each reaction:
Predict the sign of the entropy change, ΔS.
Explain your reasoning in terms of physical state and number of gaseous molecules.
(5 marks)
Reaction A:
Correctly predicts ΔS is negative. (1 mark)
States that the number of moles of gas decreases from 4 to 2. (1 mark)
Links the decrease in gaseous particles to reduced disorder or fewer possible arrangements. (1 mark)
Reaction B:
Correctly predicts ΔS is positive. (1 mark)
Explains that a gas is formed from solids, increasing particle freedom and disorder. (1 mark)
Maximum of 5 marks.

