OCR Specification focus:
‘Explain entropy as energy dispersal; greater for more disordered systems.’
Entropy explains how energy spreads and systems become disordered, helping chemists understand why reactions proceed naturally and why some arrangements of matter are favoured.
Understanding Entropy in Chemistry
Entropy is a central concept linking energy changes to the natural direction of physical and chemical processes. In chemistry, it helps explain why some reactions occur spontaneously while others do not, even when energy changes seem favourable.
At its core, entropy describes how energy is distributed within a system and how disordered that system is. Processes that allow energy to spread out more widely are associated with an increase in entropy. Conversely, processes that concentrate energy into fewer arrangements decrease entropy.
What Is Meant by Energy Dispersal?
Energy dispersal refers to the spreading out of energy among particles or throughout space. When energy becomes more widely distributed, the number of possible arrangements of particles and energy increases.
Entropy: A measure of the dispersal of energy in a system and the degree of disorder of the system.
A system with high entropy has energy spread out among many particles in many ways, whereas a system with low entropy has energy concentrated in fewer ways.
Energy dispersal can occur in several ways:
Between particles, as energy is shared among more atoms, ions, or molecules
Within particles, as energy is distributed among more vibrational, rotational, or translational motions
Into the surroundings, when energy is transferred to the environment
Each of these increases the number of possible microscopic arrangements, which corresponds to higher entropy.
Entropy and Disorder
Disorder describes how random or spread out the particles in a system are. Although entropy is not exactly the same as disorder, the two ideas are closely related and often used together at A-Level.
A more disordered system:
Has particles arranged less regularly
Allows particles to occupy more positions
Has energy spread across more possible arrangements
For example, a neatly arranged solid has low disorder and low entropy, while a gas with fast-moving particles in random positions has high disorder and high entropy.
It is important to recognise that entropy is not simply “messiness”, but a quantitative measure of how many ways energy and particles can be arranged.
Microscopic View of Entropy
From a microscopic perspective, entropy is linked to the number of possible arrangements, often called microstates.
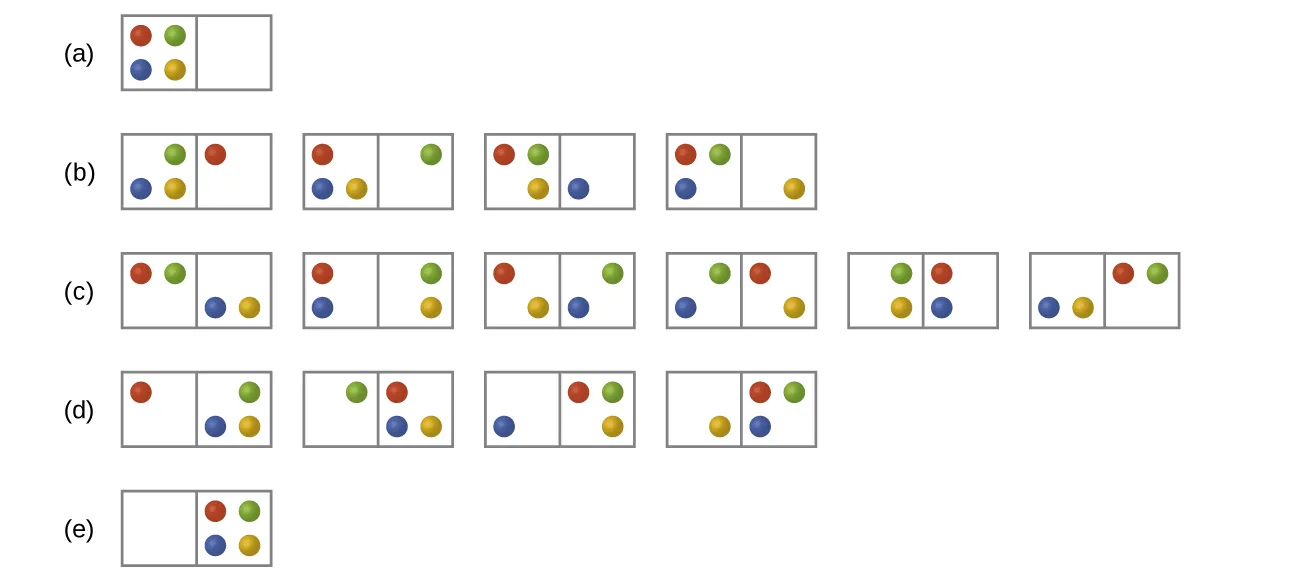
These diagrams show different microstates formed by distributing particles between compartments. More evenly spread arrangements represent higher entropy because energy can be distributed in more possible ways. Source
A system with many microstates has higher entropy because energy can be distributed in more ways.
Key ideas from the microscopic view include:
Increasing the number of particles increases the number of possible energy distributions
Increasing particle freedom of movement increases entropy
Allowing particles to spread into a larger volume increases entropy
Although OCR does not require mathematical treatment of microstates at this level, understanding this idea supports the concept of energy dispersal.
Physical Changes and Entropy
Changes of state provide clear examples of entropy changes because they involve differences in particle freedom and energy distribution.
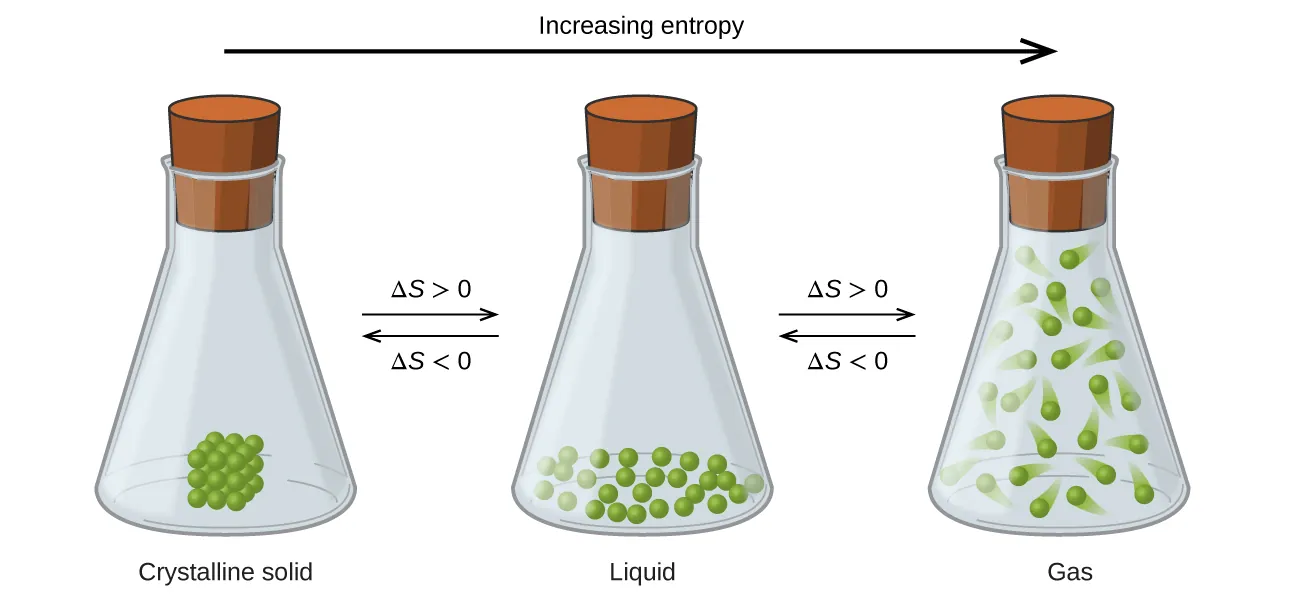
This diagram illustrates increasing disorder and energy dispersal as a substance changes from solid to liquid to gas. Greater particle freedom in gases corresponds to a much higher entropy. Source
General trends:
Solid to liquid: entropy increases due to greater particle movement
Liquid to gas: entropy increases significantly due to much greater dispersal of energy and particles
Solid to gas: very large increase in entropy
These trends occur because particles in gases have much more freedom to move and energy is spread over a larger volume.
Chemical Processes and Entropy
Chemical reactions can also involve entropy changes. Reactions that produce more particles or allow particles greater freedom generally increase entropy.
Entropy tends to increase when:
A solid or liquid forms a gas
More moles of gaseous particles are produced
Complex molecules break into simpler ones
Energy is released into the surroundings
In contrast, entropy tends to decrease when particles become more ordered or constrained, such as when gases form solids.
Entropy of the System and Surroundings
In chemistry, entropy changes are often considered for both the system and the surroundings. Energy released to the surroundings increases the entropy of the surroundings by spreading energy more widely.
Important ideas include:
Exothermic processes increase the entropy of the surroundings
Endothermic processes decrease the entropy of the surroundings
The overall direction of change depends on total energy dispersal
While later topics introduce total entropy and feasibility, this subtopic focuses on understanding entropy qualitatively as energy dispersal and disorder.
Why Entropy Increases Naturally
Natural processes tend to move towards states of higher entropy because these states allow energy to be spread out more widely. This principle explains why gases expand, why diffusion occurs, and why heat flows from hot objects to cold ones.

The diffusion of ink through water demonstrates particles spreading from a concentrated region to a more dispersed arrangement. This visual example reflects increasing disorder and energy dispersal. Source
Entropy therefore provides a unifying explanation for the direction of many physical and chemical changes, linking molecular behaviour to observable outcomes without relying on calculations at this stage.
FAQ
Entropy is linked to the number of possible microscopic arrangements (microstates) available to a system. A state with more microstates is more likely to occur naturally.
This probabilistic view explains why systems tend to move towards higher entropy without requiring intent or direction, as these states simply have more possible arrangements.
Yes, entropy can increase overall even if part of a system becomes more ordered. This happens when energy is released to the surroundings.
Local order may increase within the system
Energy transfer to the surroundings increases overall energy dispersal
Total entropy can still increase as a result
Diffusion happens because particles naturally spread from regions of high concentration to low concentration.
This spreading increases the number of possible arrangements for the particles, leading to greater energy dispersal and higher entropy, making the process spontaneous.
Increasing temperature gives particles more kinetic energy. This allows them to access more possible energy states and arrangements.
As a result, energy is dispersed among more particle motions, increasing the entropy of the system.
No, entropy applies to all physical processes, not just chemical reactions.
Examples include:
Heat flowing from hot to cold objects
Gases expanding into a vacuum
Mixing of liquids or solutions
In each case, energy and particles become more widely dispersed, increasing entropy.
Practice Questions
A chemical change involves a solid reactant forming a gaseous product.
Using the idea of entropy as energy dispersal and disorder, explain why this change is associated with an increase in entropy. You should refer to particle behaviour and energy distribution in your answer.
(5 marks)
Recognises that entropy is related to the degree of disorder or number of possible arrangements. (1 mark)
States that a gas is more disordered than a solid. (1 mark)
Explains that gas particles have greater freedom of movement than particles in a solid. (1 mark)
Links increased particle freedom to greater energy dispersal among particles and/or over a larger volume. (1 mark)
Concludes that increased disorder and energy dispersal result in an increase in entropy. (1 mark)
Explain what is meant by entropy and state how entropy changes as a system becomes more disordered.
(2 marks)
Correct definition of entropy as a measure of energy dispersal and/or disorder in a system. (1 mark)
Correct statement that entropy increases as disorder increases or as energy becomes more widely dispersed. (1 mark)

