OCR Specification focus:
‘Explain feasibility using ΔG = ΔH − TΔS and temperature; perform related calculations.’
Free energy links enthalpy and entropy to predict whether chemical reactions are thermodynamically feasible under specified conditions, helping explain why some reactions occur spontaneously while others require energy input.
Free Energy and Reaction Feasibility
Understanding reaction feasibility requires examining how enthalpy, entropy, and temperature combine to determine whether a process is thermodynamically allowed. OCR places emphasis on the quantitative use of ΔG = ΔH − TΔS, meaning students must be able to interpret the balance of energetic and entropic factors that drive chemical change.
Gibbs Free Energy
The concept of Gibbs free energy underpins predictions of thermodynamic feasibility. It links two competing effects: the tendency to minimise energy and the tendency to maximise disorder.
Gibbs Free Energy (ΔG): The energy available to do work, determining whether a reaction is thermodynamically feasible at constant temperature and pressure.
A reaction’s ΔG value indicates whether the overall process can occur without external energy. Negative ΔG corresponds to feasible (thermodynamically spontaneous) change, while positive ΔG indicates non-feasibility under those exact conditions.
The Gibbs Free Energy Equation
Students must be able to use the relationship between ΔG, ΔH, and ΔS, and interpret how temperature affects feasibility.
Gibbs Free Energy Equation (ΔG) = ΔH − TΔS
ΔG = Free energy change, determining feasibility (kJ mol⁻¹)
ΔH = Enthalpy change, heat energy transfer at constant pressure (kJ mol⁻¹)
T = Temperature in Kelvin (K)
ΔS = Entropy change, measure of disorder (J K⁻¹ mol⁻¹)
Because ΔS is usually given in J K⁻¹ mol⁻¹, students must ensure unit consistency when performing calculations. The sign and magnitude of each term determine how temperature influences ΔG.
Interpreting Feasibility
Feasibility refers solely to thermodynamic spontaneity. This does not guarantee a reaction will occur quickly, only that it is energetically allowed. OCR requires students to judge feasibility under specified temperature conditions using ΔG = ΔH − TΔS.
Key points for interpretation:
ΔG < 0 → Reaction is feasible under those conditions.
ΔG = 0 → System is at equilibrium.
ΔG > 0 → Reaction is not feasible under those conditions.
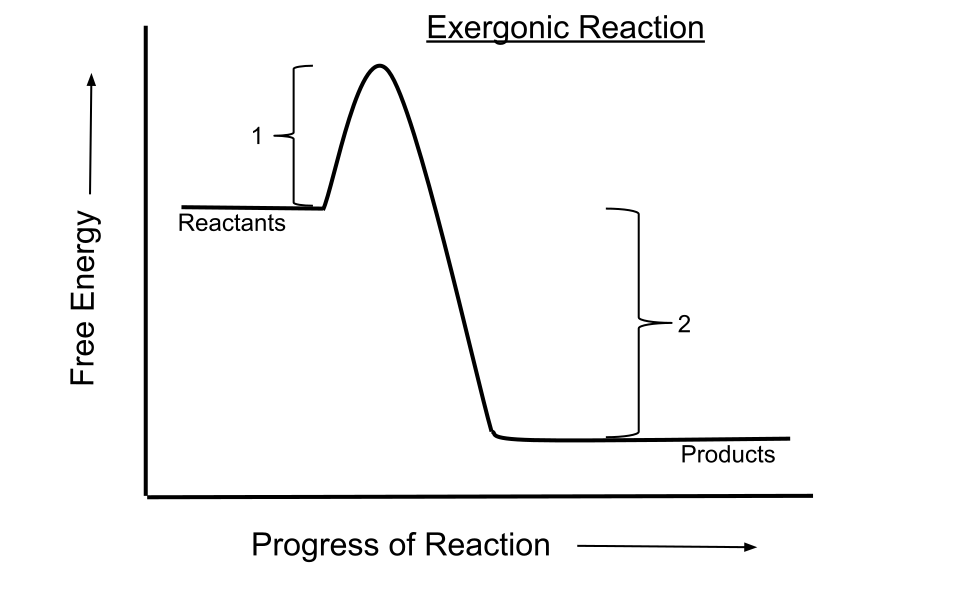
This energy profile shows an exergonic reaction where products have lower Gibbs free energy than reactants, giving a negative ΔG and thermodynamic feasibility. The activation energy peak represents kinetic detail beyond free-energy feasibility. Source
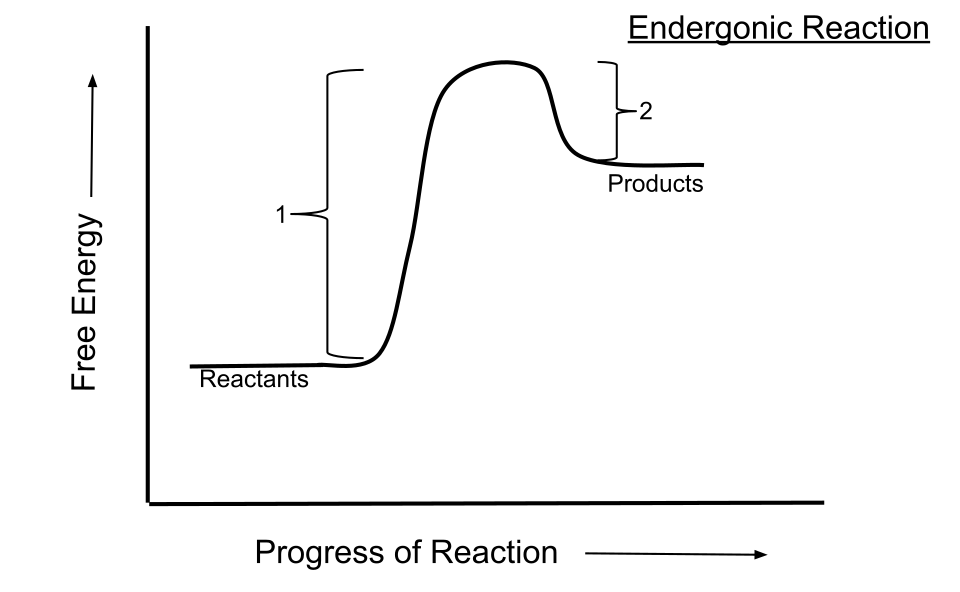
This energy profile illustrates an endergonic reaction in which products lie at higher Gibbs free energy than reactants, giving a positive ΔG and non-feasibility under these conditions. The activation energy peak shows kinetic information not required for ΔG analysis. Source
Although feasibility is linked to ΔG, kinetic considerations (e.g., activation energy) are addressed separately and do not form part of this subsubtopic.
Temperature Dependence of Feasibility
The equation shows that feasibility depends strongly on temperature. The balance between ΔH and TΔS decides whether ΔG is positive or negative.
Cases Based on Sign Combinations
Students should recognise the four important combinations of ΔH and ΔS:
ΔH negative, ΔS positive
Always feasible, because ΔH favours spontaneity and −TΔS also decreases ΔG.
ΔH negative, ΔS negative
Feasible at low temperatures because the −TΔS term becomes less positive.
ΔH positive, ΔS positive
Feasible at high temperatures when −TΔS becomes sufficiently negative.
ΔH positive, ΔS negative
Not feasible at any temperature because both terms increase ΔG.
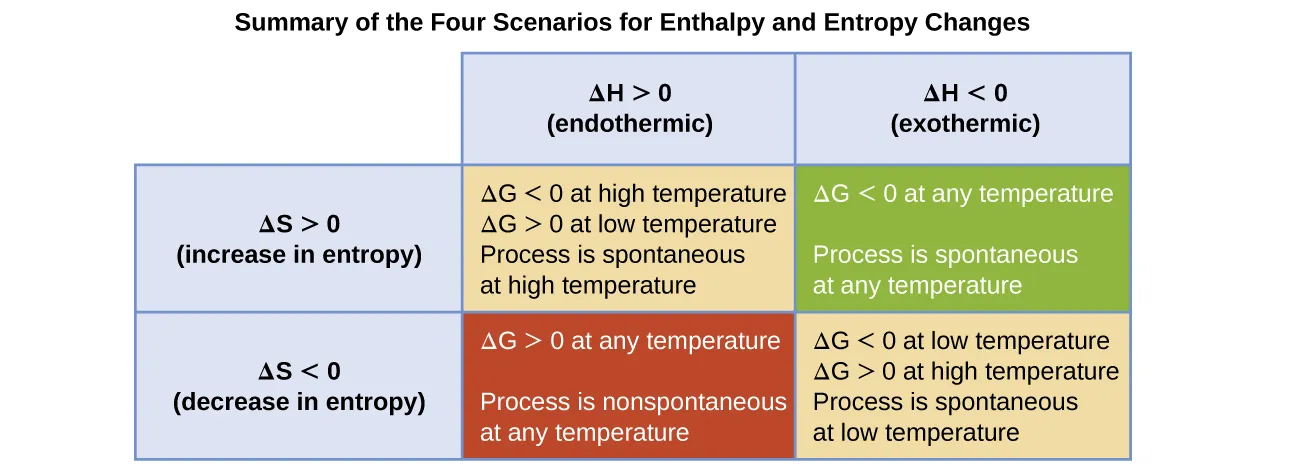
This summary grid shows how the signs of ΔH and ΔS determine the sign of ΔG and whether a reaction is feasible at all temperatures, high temperatures, or low temperatures. Temperature-dependent cases highlight the role of the −TΔS term. Source
These qualitative rules help predict feasibility even without numerical calculation.
Entropy Considerations in Free Energy
To judge feasibility, students must interpret entropy contributions correctly. Entropy reflects the dispersal of energy within a system. Changes in entropy influence the magnitude of the TΔS term.
Entropy (S): A measure of disorder or energy dispersal within a system.
Systems with increased disorder have larger entropy values. When reactions produce more gaseous particles, break ordered lattices, or increase molecular motion, ΔS often becomes positive, lowering ΔG as temperature increases.
The Role of Temperature in TΔS
Temperature directly scales the impact of entropy change. Even small entropy changes can become decisive at high temperatures because T multiplies ΔS. As temperature rises, the −TΔS term becomes more negative, favouring feasibility if ΔS is positive.
Situations where T determines spontaneity:
Positive ΔS → Higher temperature makes feasibility more likely.
Negative ΔS → Higher temperature makes feasibility less likely.
This explains why endothermic processes with favourable entropy (e.g., dissolving some ionic solids) can become feasible only at elevated temperatures.
Using ΔG to Determine Feasibility Under Specified Conditions
OCR requires students to substitute values into the Gibbs equation to assess feasibility at a given temperature. When evaluating a reaction:
Ensure ΔS is converted into kJ K⁻¹ mol⁻¹ before substituting.
Use temperature in Kelvin only.
Interpret the sign of ΔG rather than memorising outcomes.
These steps reinforce how quantitative analysis reflects energetic and entropic contributions to reaction behaviour.
Equilibrium Condition ΔG = 0
At the threshold between feasible and non-feasible conditions, ΔG = 0. This corresponds to equilibrium, where forward and reverse processes occur at equal rates. Rearranging the equation allows prediction of temperature at which feasibility changes.
Equilibrium Temperature (T) = ΔH / ΔS
T = Temperature at which ΔG becomes zero (K)
ΔH = Enthalpy change (kJ mol⁻¹)
ΔS = Entropy change (kJ K⁻¹ mol⁻¹)
One sentence is required after any equation block to maintain correct formatting. This equilibrium expression helps identify boundary temperatures where thermodynamic favourability reverses.
Feasibility Versus Observed Reaction
While ΔG predicts thermodynamic feasibility, actual reaction occurrence depends on kinetics. A thermodynamically feasible reaction may not proceed if the activation energy is large, though such kinetic constraints belong to a different subsubtopic.
These structured principles align with OCR expectations for analysing feasibility using free energy, temperature, and entropy.
FAQ
The Gibbs free energy equation includes the term TΔS, which represents how entropy contributes to feasibility. Temperature must be in Kelvin because the Kelvin scale starts at absolute zero, where molecular motion ceases.
Using Celsius would distort the magnitude of the TΔS term and give incorrect values for ΔG. Kelvin ensures proportionality between temperature and molecular energy, which is essential for meaningful thermodynamic predictions.
Yes, a reaction with a positive ΔH can be feasible if it has a sufficiently positive ΔS and occurs at a high enough temperature.
In these cases, the −TΔS term becomes large and negative, outweighing the positive ΔH. This situation is common in reactions where disorder increases significantly, such as reactions producing gases.
When ΔG equals zero, the system is at equilibrium. This means there is no net tendency for the reaction to proceed forwards or backwards.
Both directions occur at the same rate, and the concentrations of reactants and products remain constant, provided temperature and pressure do not change.
Thermodynamic feasibility refers only to whether a reaction is energetically allowed based on ΔG.
It does not consider how fast the reaction occurs. A reaction may be thermodynamically feasible but extremely slow if it has a large activation energy, making it impractical under normal laboratory conditions.
The size of ΔS determines how strongly temperature influences ΔG.
A large positive ΔS means feasibility increases rapidly with temperature
A small ΔS means temperature has a limited effect
A large negative ΔS makes high temperatures strongly unfavourable
This explains why some reactions change feasibility over relatively narrow temperature ranges.
Practice Questions
A reaction has an enthalpy change ΔH = −45 kJ mol⁻¹ and an entropy change ΔS = −120 J K⁻¹ mol⁻¹.
State whether the reaction is thermodynamically feasible at low temperatures. Give a reason for your answer.
(2 marks)
Correct statement that the reaction is feasible at low temperatures (1 mark)
Correct reasoning that ΔH is negative and the unfavourable −TΔS term is small at low temperature, so ΔG is negative (1 mark)
For a reaction, the enthalpy change ΔH is +80 kJ mol⁻¹ and the entropy change ΔS is +200 J K⁻¹ mol⁻¹.
(a) Explain how temperature affects the feasibility of this reaction using the equation ΔG = ΔH − TΔS.
(b) Determine whether the reaction is thermodynamically feasible at 500 K. Show your reasoning.
(5 marks)
(a) Explanation of temperature effect (3 marks)
Correct use of ΔG = ΔH − TΔS to describe feasibility (1 mark)
Statement that ΔS is positive so the −TΔS term becomes more negative as temperature increases (1 mark)
Conclusion that the reaction becomes feasible at sufficiently high temperatures (1 mark)
(b) Feasibility at 500 K (2 marks)
Correct handling of units by converting ΔS to kJ K⁻¹ mol⁻¹ or consistent reasoning using magnitudes (1 mark)
Correct conclusion that ΔG is negative at 500 K and the reaction is feasible (1 mark)

