OCR Specification focus:
‘Reduce aldehydes and ketones with NaBH₄ via H⁻ nucleophile and subsequent protonation to give alcohols.’
This topic explains how sodium borohydride reduces aldehydes and ketones, focusing on nucleophilic addition mechanisms, reagent conditions, and why alcohols are formed selectively.
Nucleophilic Addition to Carbonyl Compounds
Carbonyl compounds such as aldehydes and ketones undergo reactions that exploit the polarity of the C=O bond. Oxygen is more electronegative than carbon, creating a partial negative charge (δ–) on oxygen and a partial positive charge (δ+) on carbon.
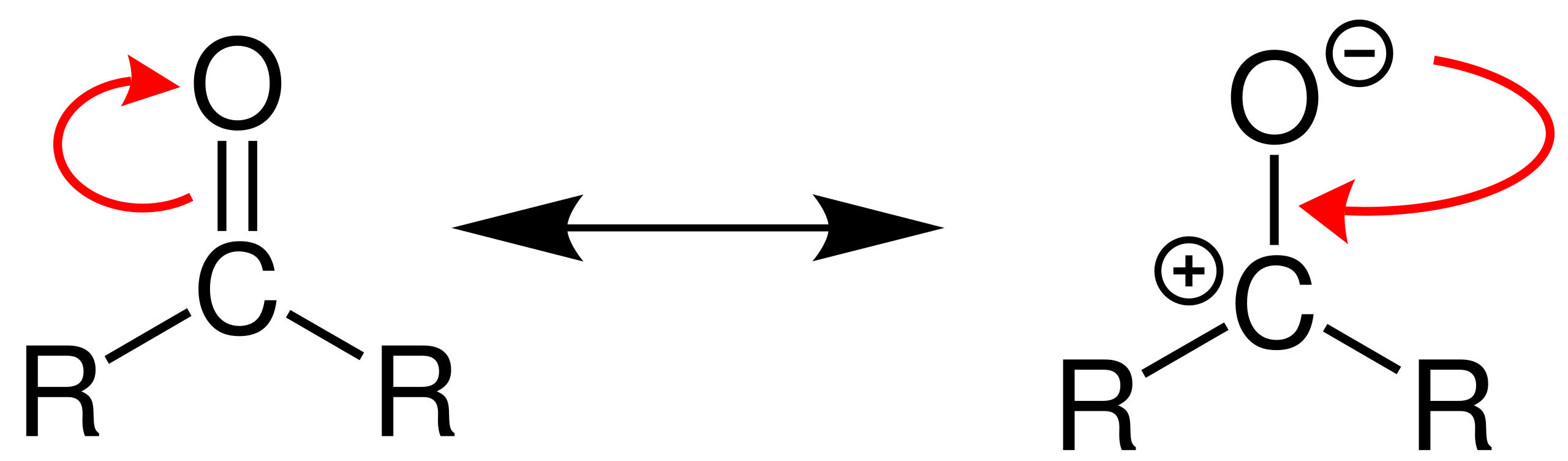
This diagram shows the polarity of the carbonyl group, with δ+ on carbon and δ− on oxygen, creating a strong dipole. The δ+ carbon is the key electrophilic site attacked during nucleophilic addition. Source
This makes the carbonyl carbon susceptible to attack by nucleophiles.
In nucleophilic addition reactions, the π bond of the carbonyl group is broken, and two new single bonds are formed. Unlike addition to alkenes, nucleophilic addition does not involve electrophiles attacking a region of high electron density; instead, electron-rich species attack an electron-deficient carbon atom.
Nucleophile: A species that donates a lone pair of electrons to form a covalent bond with an electron-deficient atom.
A clear understanding of carbonyl polarity is essential before considering reductions using sodium borohydride.
Sodium Borohydride as a Reducing Agent
Sodium borohydride (NaBH₄) is a key reducing agent used at A-Level to convert aldehydes and ketones into alcohols. It is preferred because it is selective, safe to handle, and works under mild conditions.
Reduction: A reaction involving the gain of electrons or hydrogen, or the loss of oxygen.
NaBH₄ contains the borohydride ion (BH₄⁻), which acts as the source of the nucleophile in the reaction. Each BH₄⁻ ion can, in principle, supply hydride ions (H⁻), though at this level the focus is on the delivery of a single hydride.
Unlike stronger reducing agents, sodium borohydride does not react violently with water, allowing reactions to be carried out in aqueous or alcoholic solvents such as methanol or ethanol.
The Hydride Ion and Nucleophilic Attack
The key nucleophile in NaBH₄ reductions is the hydride ion (H⁻). Although hydrogen is often associated with acidity, in this context it behaves as a nucleophile due to its high electron density.
Hydride ion (H⁻): A hydrogen atom that has gained an extra electron, making it a powerful nucleophile.
The hydride ion attacks the δ+ carbon of the carbonyl group.
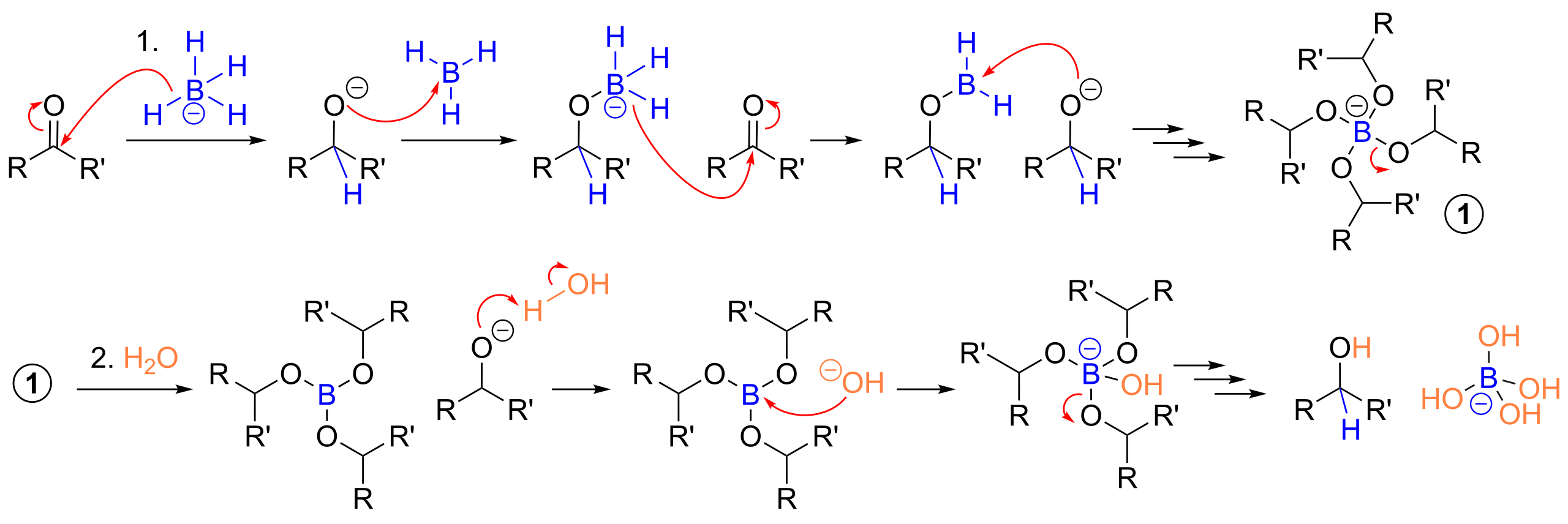
This mechanism shows hydride (H⁻) transfer from NaBH₄ to the carbonyl carbon, breaking the π bond and forming an alkoxide intermediate. A second step then protonates the alkoxide to give the alcohol product. Source
This breaks the π bond between carbon and oxygen, forming a tetrahedral intermediate with a negatively charged oxygen ion (alkoxide).
This step is the defining feature of nucleophilic addition and must be shown clearly in mechanisms, including curly arrows to represent electron movement.
Reaction Mechanism: Step-by-Step Overview
The reduction of aldehydes and ketones using NaBH₄ proceeds in two distinct stages:
Stage 1: Nucleophilic addition
H⁻ from BH₄⁻ attacks the carbonyl carbon.
The C=O π bond breaks.
An alkoxide ion (R–CHO⁻ or R₂CO⁻) is formed.
Stage 2: Protonation
The negatively charged oxygen is protonated by water or alcohol.
An alcohol is produced.
There must always be a clear separation between these two stages when describing or drawing the mechanism.
Products of NaBH₄ Reduction
The nature of the carbonyl compound determines the type of alcohol formed:
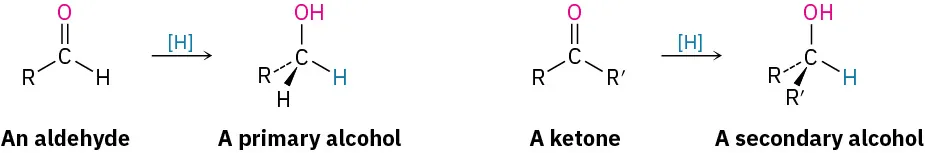
The figure contrasts reductions of aldehydes and ketones, highlighting that aldehydes give primary alcohols while ketones give secondary alcohols. The chemistry shown matches NaBH₄ reductions, although the reducing agent is represented generically as [H]. Source
Aldehydes are reduced to primary alcohols
Ketones are reduced to secondary alcohols
Carbonyl Reduction (general) = Carbonyl compound + 2[H] → Alcohol
[H] = Reducing equivalents supplied by NaBH₄ (no unit)
Aldehydes are generally more reactive than ketones because they have less steric hindrance and fewer electron-donating alkyl groups, making the carbonyl carbon more accessible to nucleophilic attack.
Reaction Conditions and Selectivity
Sodium borohydride reductions are typically carried out:
At room temperature
In aqueous ethanol or methanol
Without the need for heating or reflux
NaBH₄ selectively reduces aldehydes and ketones only. It does not reduce carboxylic acids or esters under these conditions, which is a significant advantage in multi-step organic synthesis.
This selectivity arises because aldehydes and ketones have a highly polar C=O bond, whereas carboxylic acid derivatives are stabilised by resonance and are less susceptible to nucleophilic attack.
Importance for Mechanisms and Examinations
For OCR A-Level Chemistry, students must be able to:
Identify NaBH₄ as a nucleophilic reducing agent
Explain the role of the hydride ion
Show curly arrows correctly in both steps
Predict the correct alcohol product from a given aldehyde or ketone
Precision in terminology and mechanism structure is essential, as errors often arise from confusing nucleophilic addition with electrophilic addition or oxidation reactions.
FAQ
Sodium borohydride is described as mild because it reduces aldehydes and ketones without reacting with most other functional groups.
This selectivity arises from the controlled release of hydride ions and its stability in protic solvents, such as ethanol or water.
As a result, reactions can be carried out safely at room temperature without vigorous side reactions.
After hydride addition, the intermediate formed is an alkoxide ion, which is not the final product.
A proton source, such as water or ethanol, is required to convert the alkoxide into an alcohol.
Without this protonation step, the reaction would stop at the negatively charged intermediate and no alcohol would form.
Ketones have two alkyl groups attached to the carbonyl carbon, while aldehydes have only one.
These alkyl groups both donate electron density and create steric hindrance, reducing the positive character of the carbonyl carbon.
As a result, nucleophilic attack by hydride ions occurs more slowly for ketones.
The reaction proceeds via nucleophilic addition followed by protonation, which are chemically distinct processes.
Curly arrows are used to show electron movement clearly:
From the hydride ion to the carbonyl carbon
From the C=O π bond to the oxygen
From a proton to the alkoxide oxygen
Combining these steps would incorrectly represent the reaction pathway.
Sodium borohydride is stable, easy to handle, and does not require anhydrous conditions.
It does not react violently with water, reducing risk in practical work.
Additionally, reactions proceed cleanly with minimal by-products, making results easier to observe and interpret.
Practice Questions
Sodium borohydride, NaBH₄, is used to reduce carbonyl compounds.
State the type of reaction that occurs when an aldehyde is reduced by NaBH₄ and identify the nucleophile involved.
(2 marks)
1 mark
Correctly states nucleophilic addition as the type of reaction.
1 mark
Identifies the hydride ion (H⁻) as the nucleophile.
Propanone can be reduced using sodium borohydride in aqueous ethanol.
(a) Describe the mechanism for the reduction of propanone using NaBH₄.
(b) Name the organic product formed.
(5 marks)
(a) Mechanism description (4 marks)
1 mark
States that H⁻ attacks the δ+ carbon of the C=O group.
1 mark
Describes breaking of the C=O π bond during nucleophilic attack.
1 mark
Identifies formation of a negatively charged alkoxide intermediate.
1 mark
Describes protonation of the alkoxide by water or ethanol to form the alcohol.
(b) Product identification (1 mark)
1 mark
Correctly names the product as propan-2-ol.

