OCR Specification focus:
‘Oxidise aldehydes with acidified dichromate, [O], to form carboxylic acids.’
Oxidation of aldehydes is a fundamental transformation in organic chemistry, allowing selective conversion into carboxylic acids using powerful oxidising agents under acidic conditions.
Oxidation of Aldehydes
Aldehydes undergo characteristic oxidation reactions due to the presence of the aldehyde functional group, which contains a carbonyl carbon bonded to both a hydrogen and an alkyl or aryl group. This hydrogen makes aldehydes readily oxidised, unlike ketones, whose carbonyl carbon lacks a hydrogen atom. The OCR specification highlights the ability to oxidise aldehydes with acidified dichromate, represented as [O], forming carboxylic acids, and understanding this transformation is essential for reaction pathway analysis and functional group interconversion.
The Aldehyde Functional Group
The reactivity of aldehydes in oxidation reactions originates from their structure. Aldehydes contain a carbonyl group with significant polarity, generating an electrophilic carbon that can undergo further oxidation.
Aldehyde: An organic compound containing a carbonyl group bonded to at least one hydrogen atom, typically written as R–CHO.
The presence of this carbon-bonded hydrogen is what enables further oxidation to the corresponding carboxylic acid, forming the basis of many qualitative and synthetic procedures in organic chemistry.
Acidified Dichromate as an Oxidising Agent
Acidified potassium dichromate(VI), K₂Cr₂O₇/H⁺, is the standard reagent used for oxidising aldehydes in laboratory and industrial contexts. It functions as a powerful oxidising agent, supplying oxygen to the aldehyde during the reaction and being reduced itself in the process. Its distinctive orange-to-green colour change is also widely used in practical settings, though the specification emphasises its role primarily as an oxidising species.
Key features of acidified dichromate:
It provides the oxidising species Cr₂O₇²⁻, which accepts electrons from the aldehyde.
It must be used under acidic conditions, commonly using dilute sulfuric acid.
It produces a carboxylic acid when an aldehyde is fully oxidised.
Oxidation of Aldehydes (General Reaction) = R–CHO + [O] → R–COOH
R–CHO = Aldehyde functional group
[O] = Symbol representing an oxidising agent such as acidified dichromate
R–COOH = Carboxylic acid product
This oxidation is typically heated under reflux, a technique that prevents loss of volatile aldehydes and ensures complete conversion to the carboxylic acid.
Because aldehydes are often volatile, oxidation to the carboxylic acid is typically carried out by heating the reaction mixture under reflux.
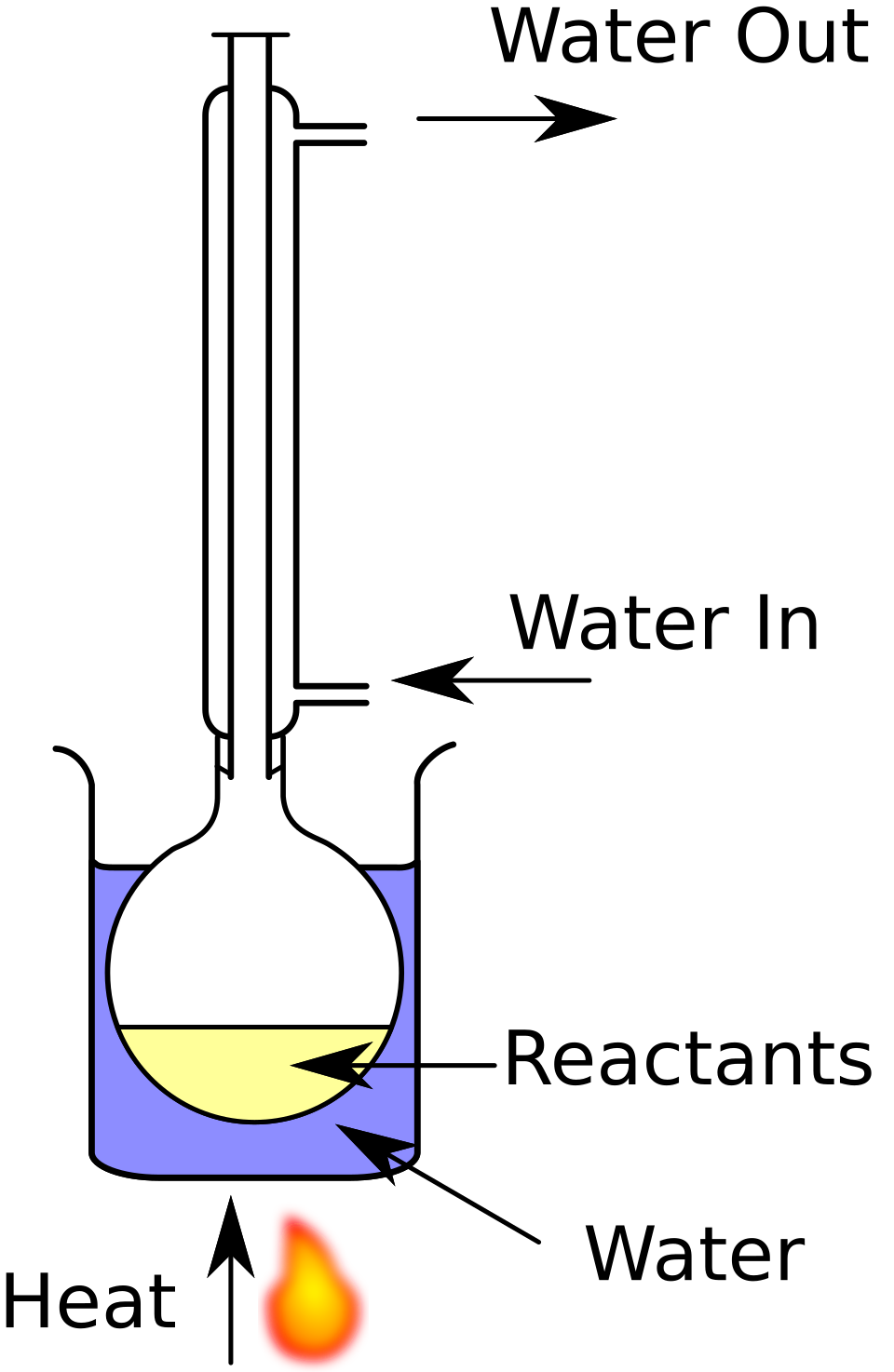
A reflux set-up allows a reaction mixture to be heated for long periods while preventing solvent and volatile organics from escaping. Vapours condense in the vertical condenser and return to the flask, maintaining a constant reaction volume. This is the standard practical condition for oxidising aldehydes to carboxylic acids using acidified dichromate. Source
Aldehydes may also be oxidised by other oxidants such as Tollens’ reagent or Fehling’s solution, although these are not the focus for this specification point, which specifically highlights acidified dichromate as the required oxidising reagent for aldehyde-to-acid conversion.
Mechanistic Overview of the Oxidation Process
Although the specification does not require full mechanistic detail, understanding the conceptual process supports deeper comprehension of aldehyde reactivity. The key idea is that the aldehyde carbonyl carbon, already partially oxidised, can undergo further oxidation because of the presence of its directly bonded hydrogen atom.
During oxidation:
The aldehyde undergoes loss of hydrogen and gain of oxygen.
The carbonyl carbon increases in its oxidation state.
The dichromate(VI) ion is reduced to chromium(III), resulting in the characteristic colour change.
This makes aldehydes distinguishable from ketones via oxidation behaviour, as ketones cannot be oxidised under similar conditions without breaking carbon–carbon bonds.
Conditions Required for Oxidation
For effective oxidation of aldehydes to carboxylic acids, several conditions must be controlled carefully:
Essential conditions:
Reflux heating to ensure aldehydes do not escape as vapour.
Use of acidified potassium dichromate(VI), which provides the required [O].
Maintaining an excess of oxidising agent to drive the reaction fully toward the carboxylic acid.
Because aldehydes are often volatile, heating under reflux is particularly important; without this setup, the aldehyde may distil out of the reaction mixture before oxidation occurs.
Observations During the Reaction
During oxidation with acidified dichromate:
The solution colour changes from orange to green as Cr₂O₇²⁻ is reduced to Cr³⁺.
A strong acidic environment remains due to the dilute sulfuric acid present.
The carboxylic acid formed often has distinctive odours, depending on its chain length.
Although observations are useful in practical work, the OCR specification emphasises the chemical transformation, not the qualitative test, as the key knowledge for this subsubtopic.
Applications of Aldehyde Oxidation
Understanding aldehyde oxidation is important not only for laboratory testing but also for reaction synthesis planning. The transformation from aldehyde to carboxylic acid enables:
Progression along synthetic pathways to produce esters, acyl chlorides, or amides from the carboxylic acid.
Identification of aldehydes in mixture analysis when necessary oxidation behaviour is compared with that of ketones.
Linking of reaction mechanisms across broader carbonyl and acid chemistry.
The overall change is R–CHO → R–COOH, and the oxidising agent is often represented by [O].
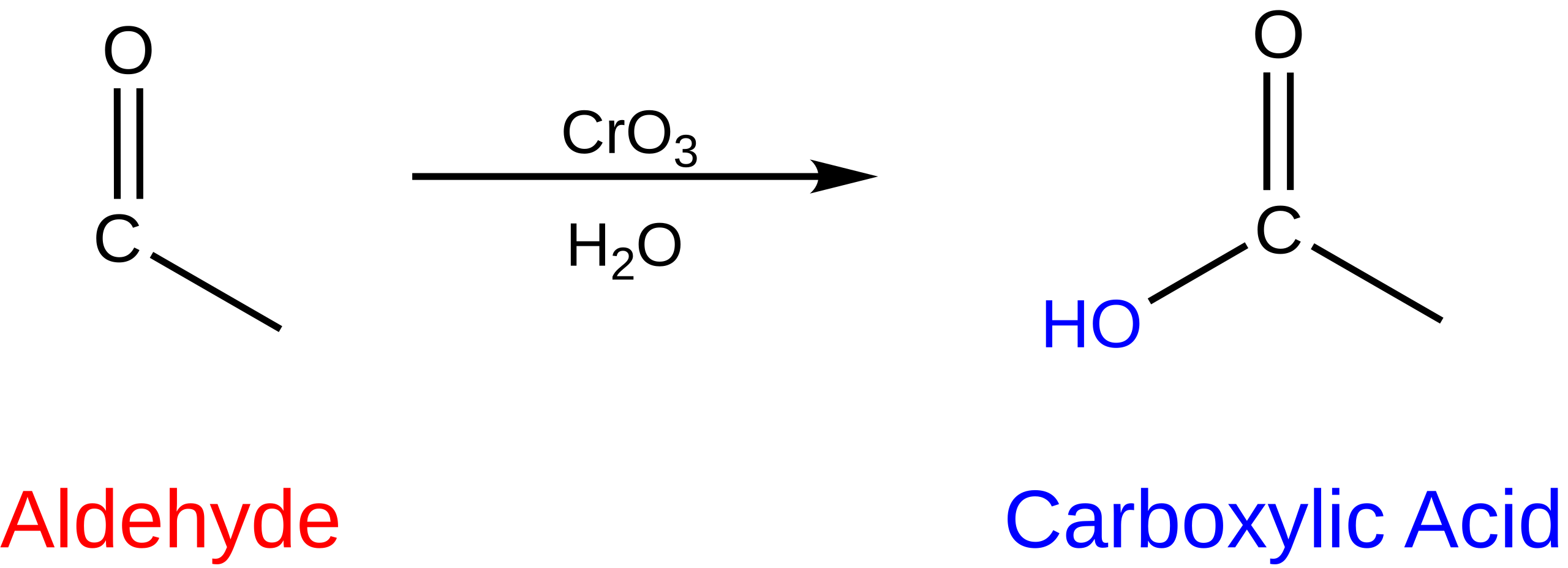
This diagram summarises the oxidation of an aldehyde functional group (–CHO) to a carboxylic acid (–COOH). The symbol [O] indicates oxygen provided by an oxidising agent such as acidified dichromate(VI). Structurally, the aldehydic hydrogen is replaced as the carbonyl carbon becomes part of the carboxyl group. Source
Under these conditions, aldehydes oxidise readily, whereas ketones are generally resistant to oxidation.
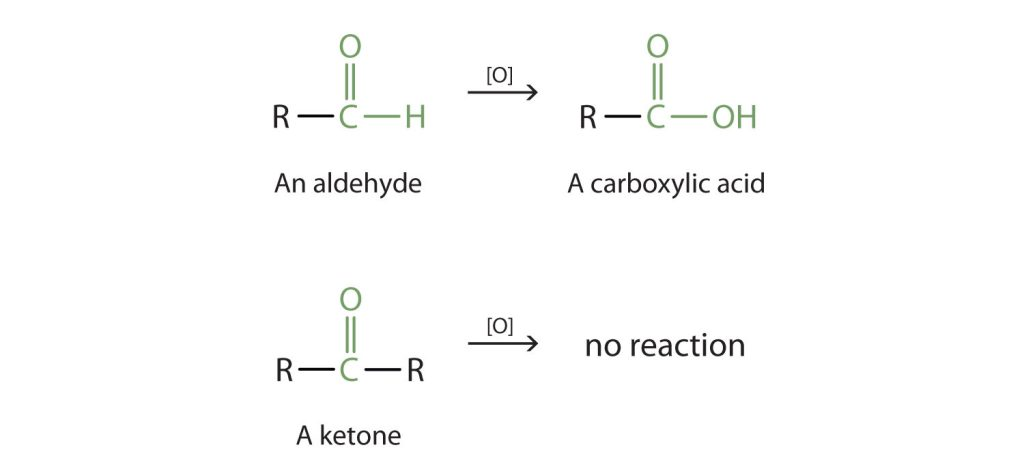
This figure contrasts aldehyde oxidation to a carboxylic acid with the lack of oxidation for a ketone under comparable conditions. Dichromate is listed as an oxidising agent consistent with OCR chemistry. Extra oxidants shown are beyond the specification but reinforce the selectivity of aldehyde oxidation. Source
Summary of Key Learning Points for This Specification Focus
Students should be able to:
Recognise that aldehydes oxidise readily due to the carbon-bonded hydrogen.
Identify acidified dichromate as the required oxidising agent denoted as [O].
Describe that the oxidation product is the carboxylic acid, formed under reflux conditions.
Write and interpret the general oxidation equation, ensuring correct functional group representation.
Explain why ketones do not undergo the same oxidation.
These concepts form the essential foundation of the OCR A-Level Chemistry understanding of aldehyde behaviour during oxidation reactions.
FAQ
Many aldehydes are volatile and have relatively low boiling points. If simple heating were used, the aldehyde could evaporate before the oxidation reaction is complete.
Reflux ensures the vapour condenses and returns to the reaction mixture, allowing prolonged heating and full oxidation to the carboxylic acid without loss of reactants.
Potassium dichromate(VI) only acts as an effective oxidising agent in acidic conditions.
The acid provides hydrogen ions that allow dichromate(VI) ions to be reduced to chromium(III), enabling electron transfer from the aldehyde during oxidation.
Aldehydes oxidise readily due to the hydrogen directly bonded to the carbonyl carbon, while most other functional groups remain unchanged under the same conditions.
This selectivity allows aldehydes to be oxidised without breaking carbon–carbon bonds or affecting alkene or aromatic structures in the molecule.
Oxidising an aldehyde to a carboxylic acid creates a versatile functional group for further reactions.
Carboxylic acids can be converted into esters, acyl chlorides, or amides, making aldehyde oxidation a useful intermediate step in multi-stage syntheses.
The colour change reflects a change in oxidation state of chromium, not the aldehyde itself.
Chromium(VI) ions are orange and are reduced to green chromium(III) ions as the aldehyde is oxidised, providing visual evidence that a redox reaction has occurred.
Practice Questions
An organic compound has the functional group R–CHO. When heated with acidified potassium dichromate(VI), the compound reacts.
(a) Name the type of reaction that occurs.
(b) State the organic product formed.
(2 marks)
(a) Oxidation – 1 mark
(b) Carboxylic acid – 1 mark
Describe how an aldehyde is oxidised using acidified potassium dichromate(VI).
Your answer should include:
The role of the oxidising agent
The conditions used
The organic product formed
One observable change during the reaction
A reason why ketones do not react under the same conditions
(5 marks)
Award marks as follows:
States that acidified potassium dichromate(VI) acts as an oxidising agent or provides [O] – 1 mark
Correct reaction conditions stated, e.g. heating under reflux / acidic conditions – 1 mark
Identifies the organic product as a carboxylic acid – 1 mark
Correct observable change stated, e.g. orange solution turns green – 1 mark
Correct explanation that ketones do not oxidise because they lack a carbon-bonded hydrogen or are resistant to oxidation under these conditions – 1 mark
Total: 5 marks

