OCR Specification focus:
‘Use 2,4-DNP to detect C=O and identify carbonyls by melting points of derivatives.’
Introduction
The 2,4-DNP test is a key qualitative method in organic chemistry for detecting the presence of the carbonyl functional group in aldehydes and ketones, providing both confirmation and identification.
The Purpose of the 2,4-DNP Test
The test using 2,4-dinitrophenylhydrazine (2,4-DNP) is a fundamental analytical technique for establishing whether an organic compound contains a C=O group. OCR requires students to understand that 2,4-DNP both detects carbonyl compounds and enables their identification through the characteristic melting points of their derivatives. The test forms an essential part of qualitative organic analysis in A-Level Chemistry.
Chemical Basis of the Test
A carbonyl group is highly reactive due to the polar C=O bond, allowing nucleophiles to attack the δ⁺ carbon. The reagent 2,4-DNP, sometimes supplied as Brady’s reagent, reacts specifically with aldehydes and ketones, forming solid derivatives known as 2,4-dinitrophenylhydrazones. These derivatives are brightly coloured, typically orange, yellow, or red, making the test visually distinctive and straightforward to interpret.
2,4-dinitrophenylhydrazine (2,4-DNP/2,4-DNPH, Brady’s reagent) reacts with aldehydes and ketones to form a 2,4-dinitrophenylhydrazone derivative.
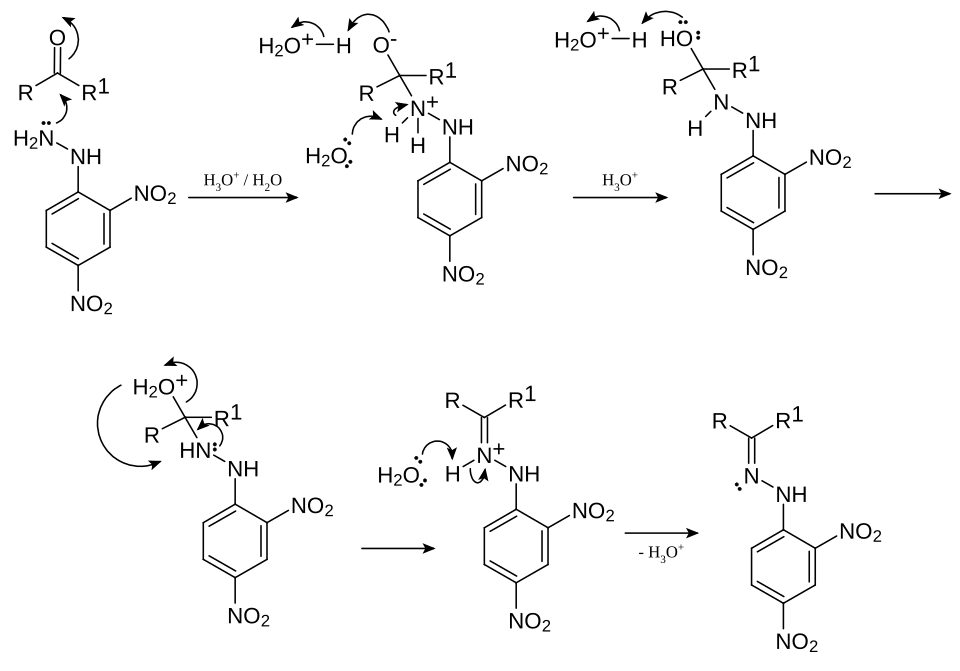
Mechanistic pathway showing conversion of a carbonyl compound into a 2,4-dinitrophenylhydrazone. This explains why a solid derivative forms in the 2,4-DNP test. The diagram also includes synthesis detail not required by the OCR syllabus. Source
What the Test Detects
The test detects the presence of:
Aldehydes (R-CHO)
Ketones (R-CO-R’)
The reaction occurs due to the nucleophilic attack on the carbonyl carbon, followed by elimination of water. This does not occur with alcohols, carboxylic acids, or esters, which lack a sufficiently electrophilic carbonyl centre or do not undergo the required addition–elimination process.
The Reaction Mechanism (Conceptual Overview)
While the OCR specification does not require students to memorise the full mechanism here, it is essential to understand the key chemical principles:
The nitrogen lone pair on the 2,4-DNP reagent attacks the electrophilic carbonyl carbon.
Proton transfers stabilise the intermediate.
Water is eliminated, forming a stable, conjugated hydrazone derivative.
This reaction is general to both aldehydes and ketones, ensuring the test is reliable across a broad range of carbonyl compounds.
Appearance of a Positive Result
A successful test produces a precipitate, which may range from yellow to deep orange. The colour variation does not carry diagnostic meaning; it simply reflects the different substituents attached to the carbonyl group. The formation of a solid is crucial, as this precipitate is used to produce a derivative with a characteristic melting point for identification.
A positive 2,4-DNP result is the formation of a yellow/orange/red precipitate, while a negative result remains as a clear yellow solution.
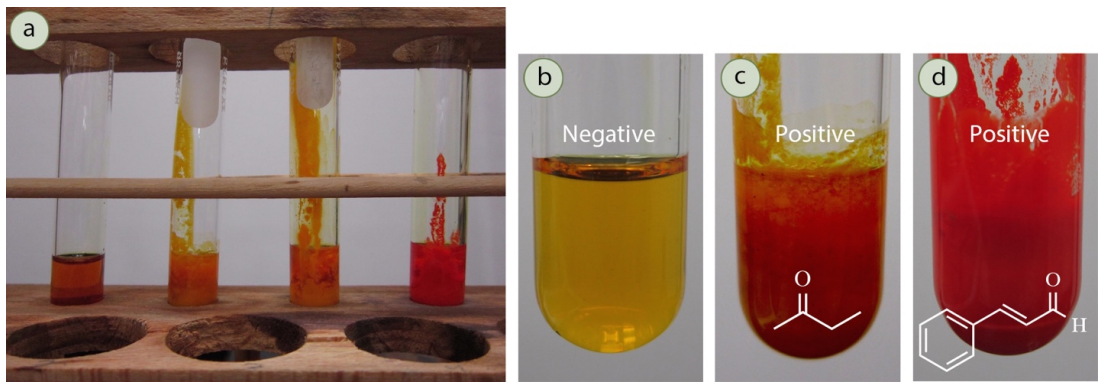
Photographs showing negative and positive outcomes of the 2,4-DNP test. A coloured precipitate indicates the presence of a carbonyl group, while a clear solution shows a negative result. Colour differences do not identify the specific carbonyl. Source
Identifying a Carbonyl Compound Using Derivatives
Although the initial test confirms the presence of a carbonyl group, OCR also requires knowledge of how melting points of derivatives allow identification.
Why Melting Points are Useful
The solid 2,4-DNP derivatives have sharp, reproducible melting points. Each aldehyde or ketone forms a unique derivative, and the melting point can be compared to a known reference value in data tables.
To identify an unknown carbonyl, the solid 2,4-DNP derivative can be purified and its melting point measured, then compared with data-book values for known derivatives.
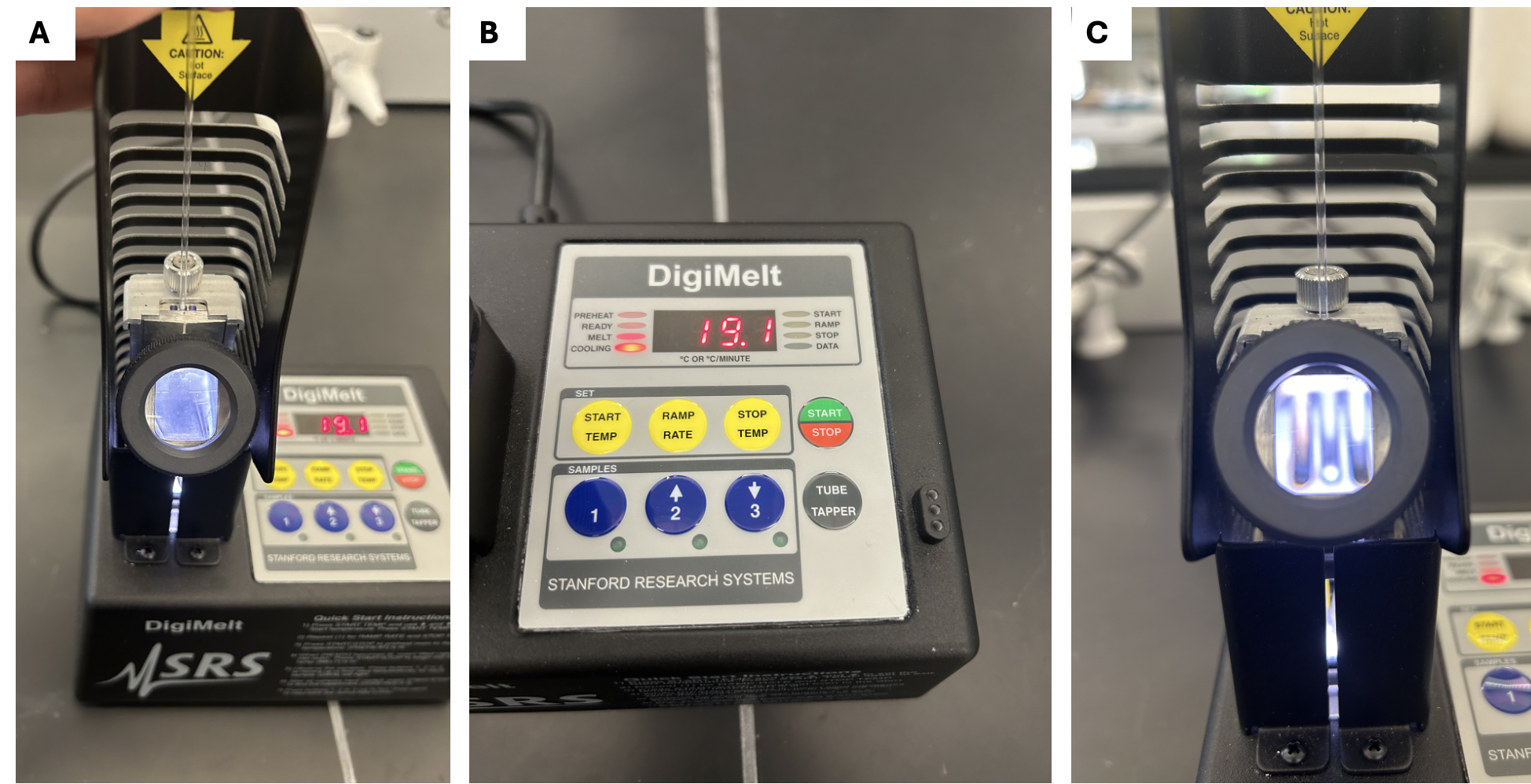
A melting point apparatus used to determine the melting range of purified 2,4-DNP derivatives. Measuring this value allows identification of carbonyl compounds by comparison with reference data. The page includes broader technique detail beyond OCR requirements. Source
Steps for Identification
Students should understand the general procedure:
Recrystallise the crude derivative to purify it.
Dry the purified crystals thoroughly.
Determine the melting point using a melting point apparatus.
Compare the measured value with a literature melting point.
This process provides a reliable match between an unknown carbonyl compound and a known reference.
Why 2,4-DNP is Selective
The selective nature of 2,4-DNP arises from its readiness to undergo condensation reactions with only aldehydes and ketones. Other functional groups either lack the reactivity or undergo different chemical pathways that do not form hydrazones.
Functional Groups That Do Not Respond
Carboxylic acids: resonance-stabilised carbonyl, less electrophilic.
Esters: require harsher conditions for nucleophilic attack.
Alcohols: no carbonyl group present.
Alkenes: no C=O group for reaction.
This specificity makes 2,4-DNP especially valuable in analysing complex organic mixtures.
Safety and Practical Considerations
Handling 2,4-DNP requires care because the compound, especially when dry, may be explosive. For this reason, it is usually supplied in a moist or acidified solution for laboratory use.
Key Practical Notes
The reagent is commonly provided dissolved in methanol with sulfuric acid.
Students must avoid contact with dry crystals.
The precipitate should be carefully filtered and recrystallised using suitable solvents.
Correct disposal procedures must be followed, as the reagent is environmentally hazardous.
Differences Between Aldehydes and Ketones in the Test
The 2,4-DNP test itself does not distinguish between aldehydes and ketones; both give positive results. Further tests, such as Tollens’ reagent, are needed to confirm the presence of an aldehyde specifically. However, for this subsubtopic, the focus remains solely on detecting and identifying carbonyls using 2,4-DNP.
Nature of the Hydrazone Derivatives
The formation of 2,4-dinitrophenylhydrazones creates highly conjugated molecules. Conjugation stabilises the derivative and is responsible for the intense colour seen in the precipitates. This conjugation also contributes to the sharp melting points used for identification in analytical chemistry.
Structural Features of the Derivative
Contains a C=N double bond.
Features two strongly electron-withdrawing nitro groups, enhancing stability.
The aromatic ring contributes to extended π-conjugation.
These molecular characteristics explain why the derivatives are robust, crystalline solids suitable for analytical work.
Advantages of the 2,4-DNP Test
Key reasons why the 2,4-DNP test remains widely used include:
High selectivity for carbonyl groups.
Clear visual confirmation through precipitate formation.
Reproducible melting points, allowing confident identification.
Compatibility with standard laboratory equipment.
The test therefore forms an essential component of organic qualitative analysis within the OCR A-Level Chemistry course.
FAQ
The reaction between 2,4-DNP and a carbonyl compound produces a large, rigid molecule with extensive conjugation.
This structure lowers solubility in the reaction solvent, causing the product to crystallise out as a solid.
The presence of two nitro groups also increases intermolecular forces, further favouring precipitation.
Impurities disrupt the crystal lattice of a solid and cause melting over a wider temperature range.
Purifying the derivative ensures a sharp melting point, which is essential for accurate identification.
Crude derivatives often melt over several degrees, making comparison with reference data unreliable.
Colour differences arise from variations in the carbonyl compound’s substituents.
These alter the extent of conjugation in the hydrazone derivative, affecting light absorption.
The colour itself has no identifying value and is not used to distinguish between aldehydes and ketones.
Dry 2,4-DNP can be unstable and potentially explosive.
Dissolving it in an acidic solvent reduces this risk and ensures safe handling in the laboratory.
The acidic medium also helps activate the carbonyl group, improving the reliability of the test.
2,4-DNP reacts selectively with aldehydes and ketones but not with most other functional groups.
This allows carbonyl compounds to be detected even in complex mixtures.
The formation of solid derivatives makes separation and further identification straightforward.
Practice Questions
A student adds 2,4-dinitrophenylhydrazine (2,4-DNP) to an unknown organic compound.
a) State the observation that indicates a positive result.
b) State what functional group is present if the result is positive.
(2 marks)
a)
Formation of a yellow, orange, or red precipitate (1 mark)
b)
Carbonyl functional group present (aldehyde or ketone) (1 mark)
A student is given an unknown organic compound and carries out a 2,4-DNP test.
a) Explain how the 2,4-DNP test is used to show that the compound contains a carbonyl group.
b) Describe how the student could use the product of the 2,4-DNP test to identify the carbonyl compound.
(5 marks)
a) (2 marks)
2,4-DNP reacts with aldehydes or ketones to form a solid derivative (1 mark)
Observation of a coloured precipitate indicates the presence of a carbonyl group (1 mark)
b) (3 marks)
The solid 2,4-DNP derivative is purified, for example by recrystallisation (1 mark)
The melting point of the purified derivative is measured (1 mark)
The measured melting point is compared with known data to identify the carbonyl compound (1 mark)

