OCR Specification focus:
‘Add CN– to carbonyls then protonate to form hydroxynitriles; outline the mechanism clearly.’
Introduction
Hydroxynitrile formation through nucleophilic addition of hydrogen cyanide is a key carbon–carbon bond-forming reaction that extends carbonyl chemistry and introduces useful synthetic functionality.
Understanding the Context of Nucleophilic Addition
Aldehydes and ketones undergo nucleophilic addition because the polar C=O bond creates an electron-deficient carbon atom. This allows negatively charged species, such as cyanide ions (CN–), to attack the electrophilic carbon and form new bonds efficiently. The reaction produces hydroxynitriles, molecules that contain both a nitrile group (–C≡N) and an alcohol group (–OH), which are important intermediates in organic synthesis.
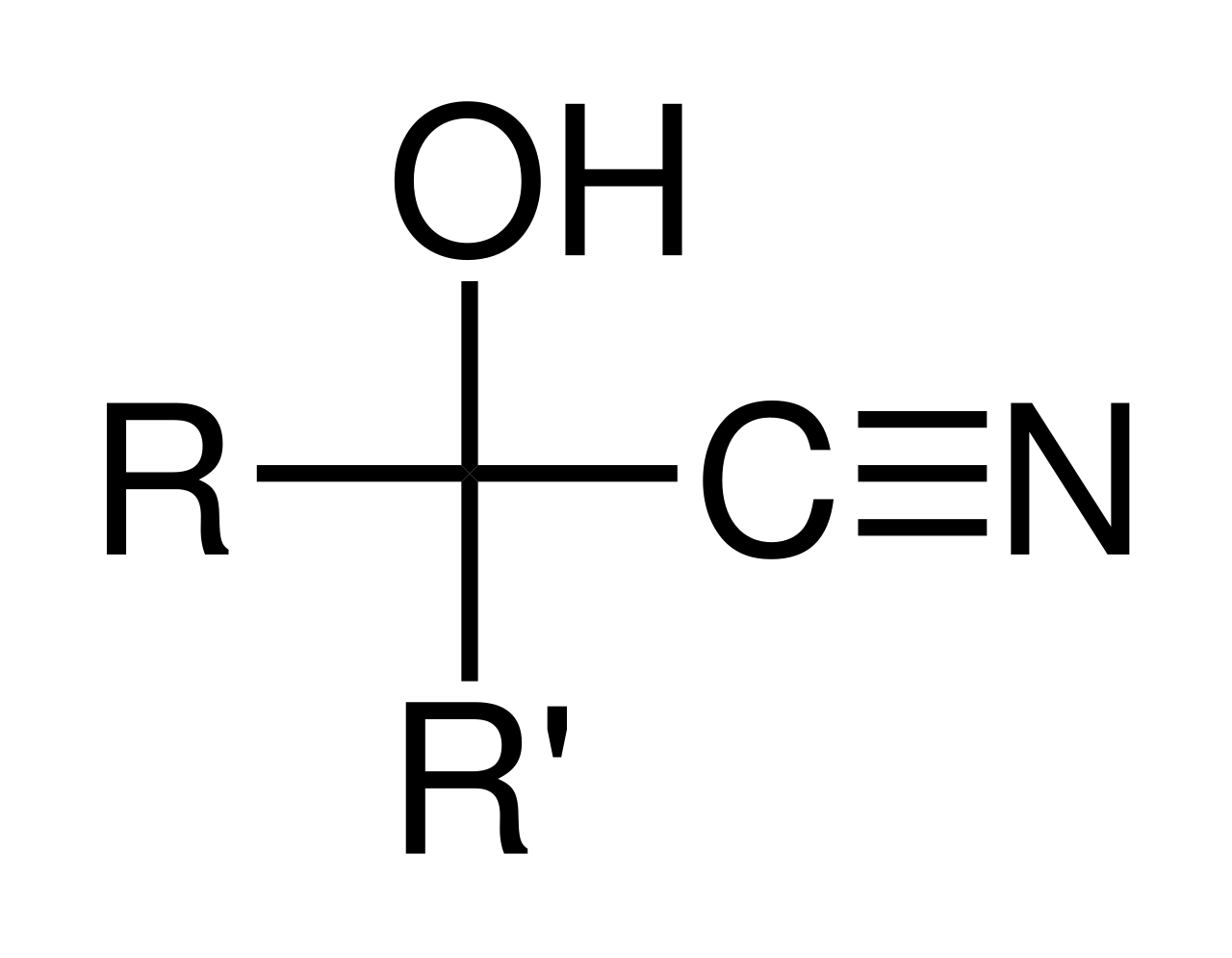
This diagram shows the general structure of an α-cyanohydrin (hydroxynitrile), where –OH and –C≡N are attached to the same carbon. The R groups represent the substituents inherited from the original aldehyde or ketone. Source
Key Features of Hydrogen Cyanide in this Reaction
Hydrogen cyanide provides both components required for hydroxynitrile formation:
The cyanide nucleophile, which attacks the carbonyl carbon
A proton source to convert the intermediate into the hydroxynitrile
However, pure HCN is hazardous and weakly dissociated, so the reaction is typically carried out using acidified sodium cyanide or potassium cyanide, which generates CN– in situ.
Structure and Reactivity of Carbonyl Compounds
The reactivity of aldehydes and ketones toward nucleophilic attack depends on both steric and electronic factors:
Aldehydes react more readily because they are less sterically hindered and the carbonyl carbon is more electrophilic.
Ketones react more slowly due to increased steric hindrance and electron-donating alkyl groups.
When CN– attacks the carbonyl group, a tetrahedral intermediate forms, which then undergoes protonation to yield the final product.
Formation of Hydroxynitriles: Reaction Mechanism
Step 1: Generation of the Nucleophile
Acidified cyanide sources generate the cyanide ion, the active nucleophile.
Nucleophile: A species that donates an electron pair to form a new covalent bond with an electron-deficient atom.
The CN– ion attacks the electron-poor carbonyl carbon.
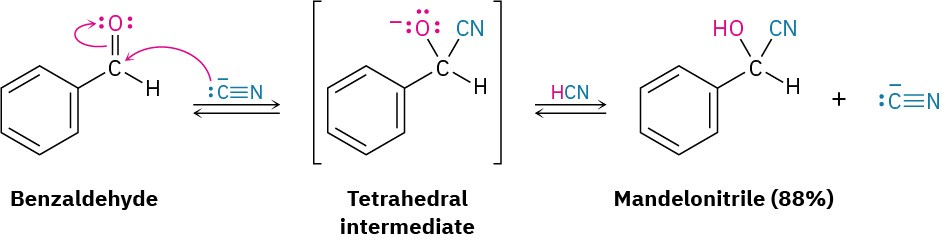
This reaction scheme illustrates cyanohydrin formation via CN⁻ attack on a carbonyl, producing a tetrahedral intermediate that is then protonated by HCN. The diagram also shows CN⁻ regeneration; the specific aromatic example and percentage yield shown go beyond the OCR syllabus. Source
A sentence separating blocks.
Step 2: Nucleophilic Attack
During nucleophilic attack, the carbonyl π-bond breaks and electron density shifts onto the oxygen atom. This produces a negatively charged alkoxide ion, a typical intermediate in nucleophilic addition reactions.
Step 3: Protonation
The alkoxide ion is protonated by an acidic species present in the reaction mixture (often HCN or dilute acid), producing the final hydroxynitrile.
Overall Reaction Description
The mechanism involves:
Attack by CN– at the carbonyl carbon
Formation of a tetrahedral intermediate
Protonation to produce the hydroxynitrile
Hydroxynitrile Formation (General Reaction): R–C(=O)–R' + HCN → R–C(OH)(CN)–R'
R, R' = Alkyl or hydrogen substituents (no units)
A normal sentence continues the explanation without introducing new equation or definition blocks.
This transformation increases molecular complexity and introduces two functional groups that can undergo further reactions, making hydroxynitrile formation synthetically valuable.
Importance of the Cyanide Ion
The cyanide ion is both a strong nucleophile and an important C–C bond-forming reagent. Its ability to attack carbonyl compounds allows the synthesis of molecules that can later be converted into:
Carboxylic acids (via hydrolysis of –C≡N)
Amines (via reduction)
Other functional groups that extend carbon frameworks
Because the reaction involves a planar carbonyl group, the CN– ion may attack from either side of the plane. This leads to the possibility of racemic mixtures when the product contains a chiral centre.
Stereochemical Outcomes
Hydroxynitriles formed from aldehydes or asymmetrical ketones often create a new stereocentre at the carbonyl carbon. Since attack by CN– is equally likely from either side, students should understand that:
A racemic mixture is usually produced
The mechanism does not favour one enantiomer over the other
Optical activity is not observed unless chiral conditions are used
These stereochemical considerations align with broader organic chemistry principles and explain why nucleophilic addition reactions are useful but not stereoselective under standard conditions.
Conditions Required for the Reaction
To ensure successful addition of HCN:
The reaction mixture must contain a source of CN–, usually NaCN or KCN
A weak acid is needed to protonate the intermediate
Temperature is controlled to minimise hazards and improve yield
Ventilation and safety measures are essential due to HCN toxicity
Safety Considerations in Laboratory Practice
Because HCN and cyanide salts are extremely toxic, school-level reactions often use alternative reagents or controlled microscale procedures. Although the specification focuses on understanding the mechanism rather than on practical execution, awareness of hazards reinforces why cyanide chemistry must be handled with care.
Summary of Mechanistic Features
Important mechanistic points include:
Polar C=O bond activates carbonyl carbon
CN– is the nucleophile that attacks
Tetrahedral intermediate is formed
Protonation yields the hydroxynitrile
Racemic mixtures arise when a chiral centre is generated
These steps reflect the OCR requirement to understand cyanide addition and to outline the mechanism clearly.
FAQ
The formation of hydroxynitriles is reversible because both the forward and reverse reactions are feasible under similar conditions.
If the concentration of CN– is low or the mixture becomes strongly acidic, the hydroxynitrile can lose CN– and reform the carbonyl compound.
This equilibrium behaviour is important in synthesis, as controlling conditions helps drive the reaction towards the desired product.
Lower temperatures favour hydroxynitrile formation by reducing the rate of the reverse reaction.
Higher temperatures increase molecular motion, making it easier for CN– to leave and regenerate the carbonyl compound.
In exam questions, mild conditions imply controlled temperatures to favour addition rather than decomposition.
Aldehydes have only one alkyl group attached to the carbonyl carbon, while ketones have two.
This results in:
Less steric hindrance in aldehydes
A more positively charged carbonyl carbon
Both factors make nucleophilic attack by CN– easier for aldehydes.
A weak acid ensures that CN– remains available as a nucleophile.
Strong acids protonate CN– to form HCN, reducing nucleophile concentration.
Using a weakly acidic environment balances nucleophile availability with efficient protonation of the intermediate.
Hydroxynitrile formation increases the carbon chain length by one carbon atom.
The nitrile group can later be transformed into other functional groups, such as:
Carboxylic acids by hydrolysis
Amines by reduction
This makes the reaction valuable for building more complex molecules step by step.
Practice Questions
Hydrogen cyanide reacts with ethanal to form a hydroxynitrile.
Explain why the cyanide ion, CN–, is able to attack the carbonyl group in ethanal.
(2 marks)
Award marks as follows:
1 mark for stating that the C=O bond is polar, making the carbonyl carbon electron-deficient / partially positive.
1 mark for stating that CN– is a nucleophile / negatively charged / has a lone pair that can attack the carbonyl carbon.
Describe the mechanism for the reaction of hydrogen cyanide with propanone to form a hydroxynitrile.
Your answer should include:
The role of the cyanide ion
The formation of any intermediate
The final protonation step
(5 marks)
Award marks as follows:
1 mark for stating that CN– acts as a nucleophile and attacks the carbonyl carbon.
1 mark for correct description of electron pair movement from CN– to the carbonyl carbon.
1 mark for stating that the C=O double bond breaks, forming a tetrahedral alkoxide intermediate.
1 mark for identifying the intermediate as negatively charged on oxygen (alkoxide ion).
1 mark for describing protonation of the alkoxide ion by HCN or an acid to form the hydroxynitrile.
Notes:
Credit clear written descriptions; curved arrows are not required in text answers.
Do not award marks for oxidation, substitution, or incorrect reaction pathways.

