OCR Specification focus:
‘Differentiate aldehydes and ketones: aldehydes oxidise to acids while Ag⁺ reduces to silver.’
Introduction
Tollens’ reagent provides a reliable method to distinguish aldehydes from ketones by exploiting their differing oxidation behaviours, producing the characteristic silver mirror in positive tests.
Tollens’ Reagent: Purpose and Chemical Basis
Tollens’ reagent is a mild, selective oxidising agent used specifically to identify aldehydes, which are readily oxidised to carboxylic acids, while ketones generally resist oxidation under these conditions. The reagent consists of ammoniacal silver ions, produced in situ, which act as the oxidant.
Tollens’ reagent: A mild oxidising agent containing the diamminesilver(I) complex, [Ag(NH₃)₂]⁺, used to distinguish aldehydes from ketones.
Aldehydes are oxidised because they contain a hydrogen atom directly bonded to the carbonyl carbon, making them more susceptible to oxidation than ketones, which lack this feature. When the aldehyde is oxidised, silver ions in the reagent are reduced to metallic silver, producing the classic mirror-like coating inside the test tube.
Formation of Tollens’ Reagent
Preparing the Diamminesilver(I) Complex
Tollens’ reagent must be freshly prepared to ensure accuracy and safety.
Aqueous silver nitrate is mixed with sodium hydroxide, forming a brown precipitate of silver(I) oxide.
Aqueous ammonia is then added dropwise until the precipitate dissolves.
The resulting solution contains the complex ion [Ag(NH₃)₂]⁺, the active species.
Diamminesilver(I) complex: A linear silver(I) complex containing two ammonia ligands, responsible for Tollens’ reagent’s oxidising ability.
This complex is a mild oxidising agent with sufficient strength to oxidise aldehydes but not ketones, aligning with the OCR requirement to differentiate between the two carbonyl classes using oxidation behaviour.
A sentence is required between definition blocks before proceeding.
Formation of Tollens’ Reagent:
Ag₂O(s) + 4NH₃(aq) + H₂O(l) → 2[Ag(NH₃)₂]⁺(aq) + 2OH⁻(aq)
[Ag(NH₃)₂]⁺ = Diamminesilver(I) complex, the oxidising agent (mol dm⁻³)
OH⁻ = Hydroxide ion, increases basicity (mol dm⁻³)
Oxidation of Aldehydes in the Tollens’ Test
Redox Chemistry
The central reaction in the Tollens’ test involves the oxidation of an aldehyde to a carboxylic acid and the simultaneous reduction of silver(I) ions to metallic silver.
The aldehyde donates electrons, undergoing oxidation.
Silver(I) ions gain electrons, being reduced to silver metal.
This reduced silver forms a thin, reflective layer, giving the distinctive silver mirror.
A positive Tollens’ test is the formation of a shiny silver mirror (Ag(s)) on the inside of a warm, clean test tube.

A positive Tollens’ test produces a reflective coating of silver metal on the inner surface of the test tube. This forms when Ag⁺ is reduced to Ag(s) as the aldehyde is oxidised. Source
Oxidation of an Aldehyde (General Reaction):
R–CHO + 2[Ag(NH₃)₂]⁺ + 3OH⁻ → R–COO⁻ + 2Ag(s) + 4NH₃ + 2H₂O
R–CHO = Aldehyde functional group
R–COO⁻ = Carboxylate ion formed upon oxidation
Ag(s) = Metallic silver forming the silver mirror
This reaction clearly demonstrates the OCR-specified requirement: aldehydes oxidise to acids, while Ag⁺ is reduced to metallic silver, allowing easy visual identification.
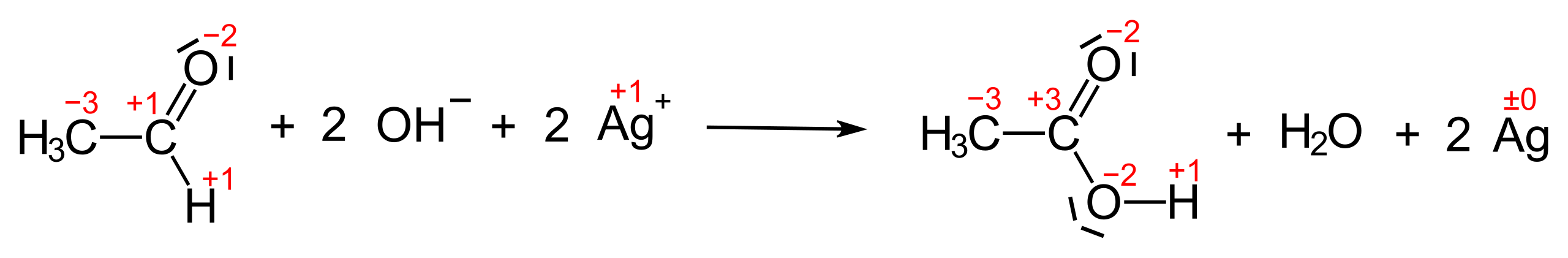
This reaction scheme summarises the redox process in Tollens’ test, showing oxidation of an aldehyde and reduction of Ag(I) to metallic silver. It focuses on the overall transformation rather than mechanistic detail. Source
Why Ketones Give a Negative Result
Lack of an Oxidisable Hydrogen
Ketones do not contain a hydrogen atom bonded to the carbonyl carbon, preventing oxidation under Tollens’ conditions. Their resistance to the reagent ensures:
No reduction of Ag⁺.
No silver mirror formation.
Reliable differentiation from aldehydes.
Oxidisable hydrogen: A hydrogen atom attached to a carbonyl carbon that can be removed during oxidation, present in aldehydes but absent in ketones.
This structural difference is fundamental and underpins the selectivity of the Tollens’ test.
Practical Considerations in the Tollens’ Test
Conditions and Safety
Tollens’ reagent must be used immediately after preparation because standing solutions may form explosive silver compounds such as silver fulminate. Good laboratory practice includes:
Preparing only the amount needed.
Avoiding storage of unused reagent.
Rinsing all glassware thoroughly after use.
Test Procedure
When conducting the Tollens’ test, students should note the following points for reliable results:
Warm the reaction mixture gently in a water bath rather than heating directly.
Ensure the reagent is clear and colourless before use (a cloudy suspension indicates incomplete dissolution of silver oxide).
Observe changes carefully:
Positive test: formation of a shiny silver mirror on the glass.
Negative test: no visible change.
Applications and Importance
Identifying Aldehydes in Organic Analysis
The Tollens’ test remains valuable in qualitative organic analysis due to its:
High selectivity for aldehydes
Visual clarity
Ability to complement other carbonyl tests, such as 2,4-DNP, which detects both aldehydes and ketones
Its use also reinforces key redox concepts important across the A-Level Chemistry course, including electron transfer, oxidation states, and functional group behaviour.
Biological Relevance
Some biologically important molecules, such as reducing sugars, can also give a positive Tollens’ test due to their ability to interconvert to aldehyde-containing forms, demonstrating that the test extends beyond simple carbonyl identification in synthetic chemistry.
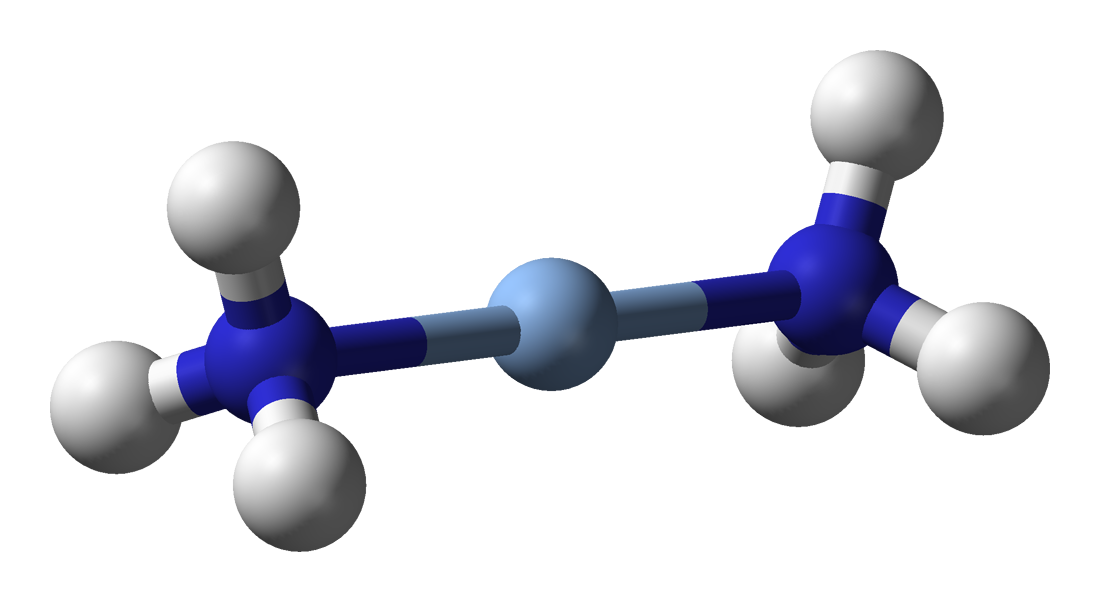
This molecular model represents the diamminesilver(I) complex, [Ag(NH₃)₂]⁺, which is the active oxidising species in Tollens’ reagent. The structural detail goes slightly beyond OCR requirements but supports understanding of the reagent as a complex ion. Source
FAQ
Tollens’ reagent is unstable and decomposes on standing. If left unused, it can form silver-containing compounds that are hazardous.
Fresh preparation ensures:
Reliable oxidation of aldehydes
Clear observations during the test
Safe disposal after use
Old reagent should never be stored, as drying residues can become shock-sensitive.
The reaction requires alkaline conditions because the aldehyde is oxidised to a carboxylate ion, not a free carboxylic acid.
The alkaline environment:
Stabilises the diamminesilver(I) complex
Allows oxidation without breaking C–C bonds
Prevents further unwanted side reactions
Acidic conditions would destroy the active silver complex.
Silver metal forms directly on the glass surface of the test tube rather than precipitating into solution.
This happens because:
Silver atoms nucleate on clean glass
The reduction occurs at the tube wall
Gentle warming promotes even deposition
Dirty or scratched glass often leads to a grey suspension instead of a mirror.
Yes, some compounds other than simple aldehydes can reduce silver(I) ions.
Examples include:
Reducing sugars (due to aldehyde formation in solution)
Certain α-hydroxy ketones
However, for OCR A-Level purposes, the test is treated as selective for aldehydes.
Tollens’ reagent is mild and selective, making it ideal for distinguishing aldehydes from ketones.
Advantages include:
No oxidation of ketones
No cleavage of carbon chains
Clear visual outcome
Stronger oxidants, such as acidified dichromate, are less selective and may oxidise multiple functional groups.
Practice Questions
Tollens’ reagent can be used to distinguish between aldehydes and ketones.
a) State the observation when an aldehyde reacts with Tollens’ reagent.
b) State why a ketone does not give the same result.
(2 marks)
a)
Formation of a silver mirror or deposit of silver metal (1 mark)
b)
Ketones are not oxidised by Tollens’ reagent / ketones lack an oxidisable hydrogen on the carbonyl carbon (1 mark)
Tollens’ reagent contains ammoniacal silver(I) ions and acts as a mild oxidising agent.
Describe how Tollens’ reagent is used to identify an aldehyde and explain the chemistry involved in the test. Your answer should include:
the change to the aldehyde
the change to the silver ions
the observable result
(5 marks)
Aldehyde is oxidised to a carboxylic acid or carboxylate ion (1 mark)
Silver(I) ions are reduced to silver metal (1 mark)
Clear reference to oxidation and reduction / redox process (1 mark)
Formation of a silver mirror or silver coating as the observation (1 mark)
Correct link between the chemical changes and the observation (e.g. silver metal forming on the test tube) (1 mark)
Maximum 5 marks

