OCR Specification focus:
‘Form substituted aromatic C–C bonds using haloalkanes or acyl chlorides with halogen carriers.’
Friedel–Crafts reactions are essential electrophilic substitution processes that allow carbon–carbon bond formation on aromatic rings, playing a central role in synthetic organic chemistry.
Overview of Friedel–Crafts Reactions
Friedel–Crafts alkylation and acylation are reactions in which an aromatic ring, typically benzene, reacts with an electrophile to form a new carbon–carbon bond. Both reactions are examples of electrophilic substitution, where a hydrogen atom on the aromatic ring is replaced by a carbon-containing group.
These reactions are significant because they enable the direct functionalisation of aromatic compounds, expanding carbon frameworks during synthesis. The reactions require halogen carriers, which are catalysts that generate strong electrophiles from relatively unreactive starting materials.
Aromatic Electrophilic Substitution
In Friedel–Crafts reactions, the aromatic π system acts as a nucleophile, donating electron density to an electrophile. This temporarily disrupts aromaticity, which is then restored by loss of a proton.
Electrophilic substitution: A reaction where an electrophile replaces a hydrogen atom in an aromatic ring, with aromaticity restored after substitution.
The stability of the benzene ring means that strong electrophiles are required. These are generated in situ using halogen carriers such as aluminium chloride (AlCl₃) or iron(III) chloride (FeCl₃).
Friedel–Crafts Alkylation
Friedel–Crafts alkylation introduces an alkyl group onto an aromatic ring using a haloalkane and a halogen carrier.
Reaction Conditions and Reagents
Aromatic compound (commonly benzene)
Haloalkane (e.g. chloromethane)
AlCl₃ or FeCl₃ as a halogen carrier
Anhydrous conditions to prevent catalyst deactivation
In Friedel–Crafts alkylation, benzene reacts with a haloalkane in the presence of a halogen carrier (typically anhydrous AlCl₃) to form an alkylbenzene.
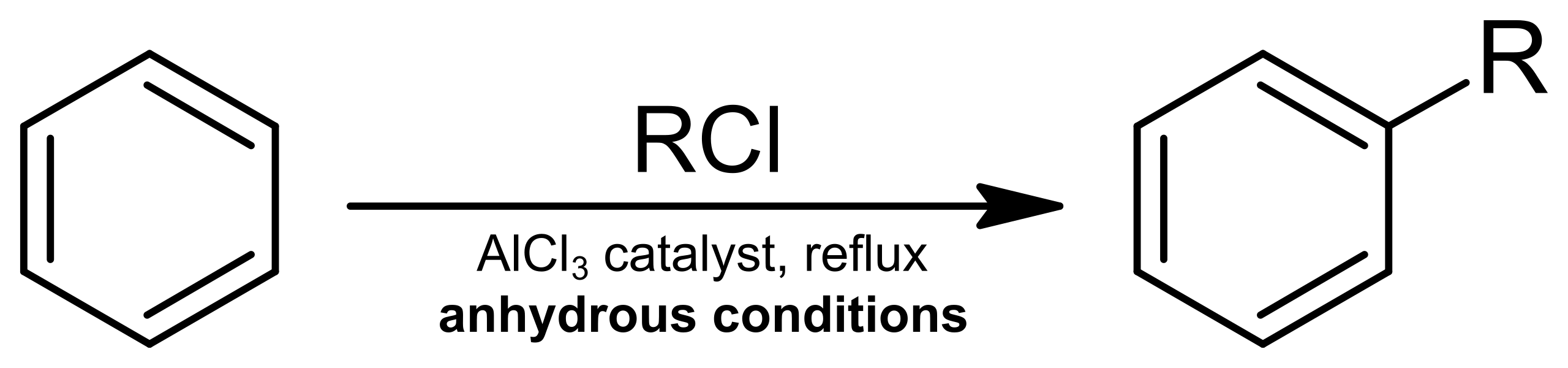
This diagram summarises Friedel–Crafts alkylation, where benzene undergoes electrophilic substitution with an alkyl halide in the presence of AlCl₃ to form an alkyl-substituted aromatic compound. Source
The halogen carrier reacts with the haloalkane to generate a positively charged electrophile.
Halogen carrier: A Lewis acid catalyst that accepts a lone pair to generate a strong electrophile from a haloalkane or acyl chloride.
At least one normal sentence must separate definition blocks, and here the key idea is that AlCl₃ acts as a Lewis acid by accepting an electron pair from the halogen.
Formation of the Electrophile
The halogen carrier polarises the C–X bond
A carbocation or carbocation-like species forms
This electrophile attacks the aromatic ring
Limitations of Alkylation
Friedel–Crafts alkylation has important limitations that restrict its synthetic usefulness:
Carbocation rearrangement may occur, producing unexpected products
Alkyl groups activate the benzene ring, leading to multiple substitutions
The reaction fails on rings containing strongly electron-withdrawing groups
These limitations are a key reason why alkylation is often replaced by acylation in multi-stage synthesis.
Friedel–Crafts Acylation
Friedel–Crafts acylation introduces an acyl group (RCO–) onto an aromatic ring using an acyl chloride.
Reaction Conditions and Reagents
Aromatic compound (e.g. benzene)
Acyl chloride (e.g. ethanoyl chloride)
AlCl₃ catalyst
Dry conditions
In Friedel–Crafts acylation, benzene reacts with an acyl chloride (RCOCl) and a halogen carrier such as anhydrous AlCl₃ to form an aromatic ketone.
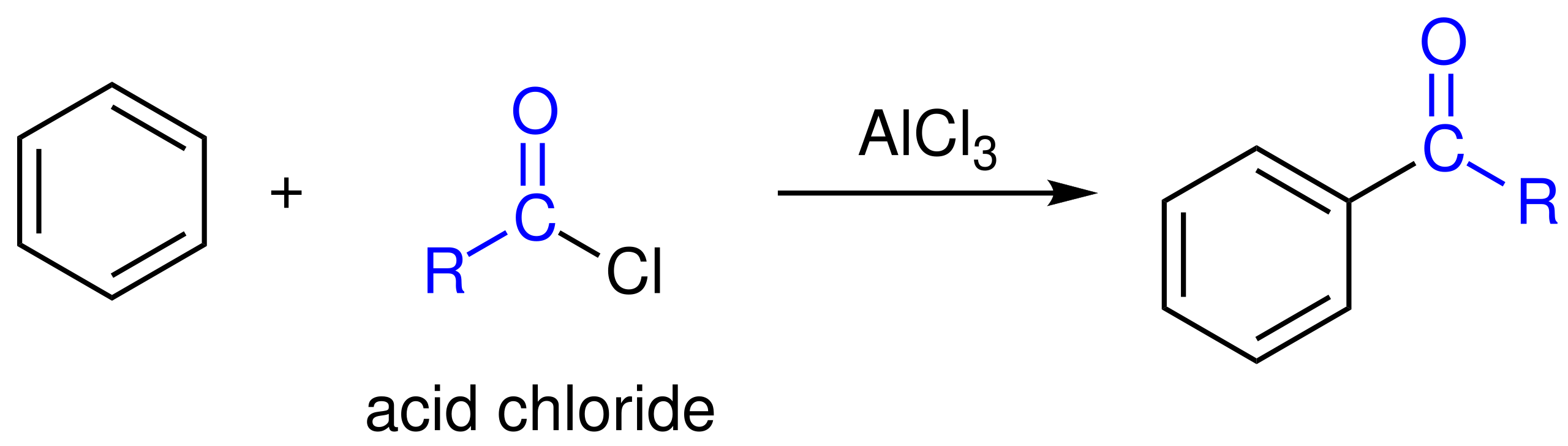
This reaction scheme shows Friedel–Crafts acylation, where an acyl group is introduced onto benzene using an acyl chloride and AlCl₃, producing an aromatic ketone via electrophilic substitution. Source
The halogen carrier reacts with the acyl chloride to form a highly stable electrophile known as an acylium ion.
Acylium ion: A positively charged ion, RCO⁺, stabilised by resonance, acting as the electrophile in Friedel–Crafts acylation.
This ion does not rearrange, making acylation more predictable than alkylation.
Key Features of Acylation
Only one substitution occurs
No carbocation rearrangement
The acyl group deactivates the ring, preventing further substitution
These features make Friedel–Crafts acylation particularly valuable in controlled synthetic routes.
Mechanism Overview (Non-Detailed)
While detailed mechanisms are not required, students should understand the general stages common to both reactions:
Generation of a strong electrophile using a halogen carrier
Electrophilic attack on the aromatic π system
Temporary loss of aromaticity
Removal of a proton to restore aromatic stability
In acylation, the electrophile is an acyl cation (acylium ion) formed from an acyl chloride with AlCl₃, and this then substitutes onto the aromatic ring.
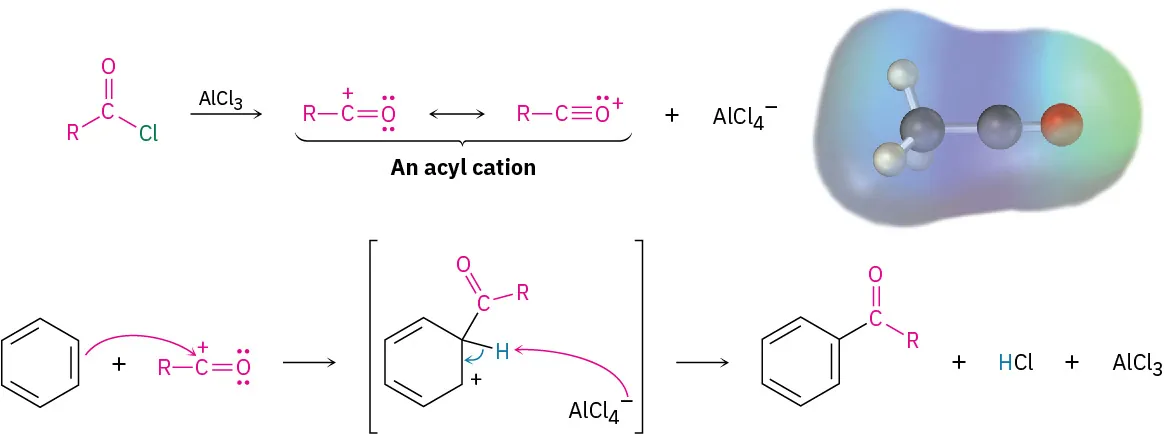
This figure outlines the Friedel–Crafts acylation mechanism, including formation of the resonance-stabilised acyl cation electrophile and its substitution onto the benzene ring. The electrostatic potential detail exceeds OCR requirements but emphasises the electron-poor carbonyl carbon. Source
Understanding these steps supports prediction of reaction outcomes and limitations.
Role of Halogen Carriers
Halogen carriers are Lewis acids, meaning they accept lone pairs of electrons. Their role is catalytic but essential, as neither haloalkanes nor acyl chlorides are sufficiently reactive alone.
Common halogen carriers include:
AlCl₃ (most commonly examined)
FeCl₃
These catalysts must remain dry, as water reacts with them and prevents electrophile formation.
Comparison of Alkylation and Acylation
Key contrasts between the two Friedel–Crafts reactions include:
Alkylation uses haloalkanes; acylation uses acyl chlorides
Alkylation can cause rearrangements; acylation cannot
Alkylation often gives multiple substitutions; acylation gives one
Acylation products can be reduced later to alkylbenzenes
This comparison is essential when selecting reactions in synthetic design.
Importance in Organic Synthesis
Friedel–Crafts reactions are fundamental tools for carbon–carbon bond formation on aromatic rings. They allow chemists to build more complex molecules from simple aromatics and are frequently used as steps within longer synthetic pathways.
Their inclusion in the OCR specification reflects their importance in understanding how aromatic chemistry supports broader organic synthesis strategies.
FAQ
Halogen carriers such as aluminium chloride react readily with water.
If moisture is present, the catalyst is hydrolysed, forming aluminium hydroxide and hydrogen chloride. This prevents formation of the electrophile and stops the Friedel–Crafts reaction from occurring.
Dry conditions are therefore essential to ensure the halogen carrier remains active throughout the reaction.
Benzene rings containing strongly electron-withdrawing groups are much less reactive.
Examples include:
Nitro (–NO₂)
Trifluoromethyl (–CF₃)
Carbonyl-containing groups
These groups reduce electron density in the ring, making electrophilic substitution energetically unfavourable or impossible under Friedel–Crafts conditions.
Aluminium chloride has an incomplete outer electron shell.
It accepts a lone pair from a halogen atom in a haloalkane or acyl chloride, forming a complex. This interaction polarises the bond and leads to formation of a strong electrophile required for substitution.
This behaviour matches the Lewis definition of an acid as an electron-pair acceptor.
The electrophile in acylation is an acylium ion.
This ion is stabilised by resonance between the carbonyl carbon and oxygen. Because of this stabilisation, it does not undergo rearrangement in the way carbocations formed during alkylation can.
As a result, the position and structure of the product are more predictable.
These reactions rely on the stability of an aromatic π system.
Aromatic rings can temporarily lose aromaticity during electrophilic attack and then regain it, releasing energy. Non-aromatic compounds do not gain this stabilisation and therefore do not undergo Friedel–Crafts substitution under similar conditions.
Practice Questions
Benzene reacts with chloromethane in the presence of anhydrous aluminium chloride.
(a) Name the type of reaction that occurs.
(b) State the role of aluminium chloride in this reaction.
(2 marks)
(a) Electrophilic substitution
1 mark
(b) Acts as a halogen carrier or Lewis acid
Accept: accepts a lone pair / generates a strong electrophile
1 mark
Benzene can be converted into an aromatic ketone using a Friedel–Crafts acylation reaction.
(a) Identify the reagent required, other than benzene, to introduce an acyl group.
(b) Explain why Friedel–Crafts acylation normally results in only one substitution on the benzene ring.
(c) State one advantage of Friedel–Crafts acylation compared with Friedel–Crafts alkylation.
(5 marks)
(a) Acyl chloride
Accept: named example such as ethanoyl chloride
1 mark
(b) Any two of the following, one mark each:
The acyl group deactivates the benzene ring
The acyl group withdraws electron density from the ring
The ring becomes less reactive towards further electrophilic substitution
Maximum 2 marks
(c) Any two of the following, one mark each:
No carbocation rearrangement occurs
Only one substitution occurs
Reaction gives a more predictable product
Produces an aromatic ketone that can be reduced later
Maximum 2 marks

