OCR Specification focus:
‘Determine numbers of protons, neutrons and electrons for atoms and ions from atomic number, mass number and any ionic charge.’
Introduction
Understanding how to determine the numbers of protons, neutrons, and electrons in atoms and ions is essential for describing chemical behaviour. This section explains their relationships clearly.
Structure of the Atom
Atoms consist of three fundamental subatomic particles: protons, neutrons, and electrons, each contributing differently to atomic properties and chemical reactions. These particles are arranged in the nucleus and surrounding shells, and their numbers define the identity, mass, and charge of atoms and ions.
Subatomic Particles and Their Roles
Protons are positively charged particles found in the nucleus and determine the identity of an element.
Neutrons are neutral particles that add mass and contribute to the stability of the nucleus.
Electrons are negatively charged particles located in shells around the nucleus and influence chemical bonding and ion formation.
Protons and neutrons are packed into the tiny central nucleus, while electrons occupy surrounding shells or energy levels.
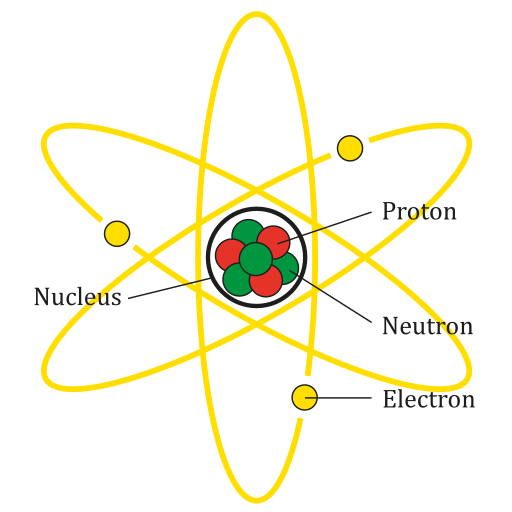
Diagram of an idealised atom showing a dense central nucleus made of protons and neutrons, surrounded by electrons in circular paths. The labels reinforce the locations of each subatomic particle used when counting protons, neutrons and electrons in atoms and ions. The orbits shown are simplified and include more structural detail than required by the OCR specification. Source
Proton: A positively charged subatomic particle located in the nucleus; it determines an element’s atomic number.
The number of protons is fixed for any given element, meaning all atoms of the same element share the same atomic number.
Neutron: A neutral subatomic particle found in the nucleus; it contributes to the mass number and nuclear stability.
Atoms with different numbers of neutrons but the same number of protons are isotopes. These differences do not affect charge but influence nuclear stability.
Electron: A negatively charged subatomic particle found in shells around the nucleus; it determines chemical interactions and the charge of ions.
Electron arrangements can vary significantly between atoms and ions. A single sentence of explanation ensures clarity before moving to numerical relationships.
Atomic Number and Mass Number
The atomic number identifies the number of protons in an atom. The mass number represents the total number of protons and neutrons in the nucleus. Together, these values allow us to determine particle numbers accurately.
Key Numerical Relationships
Atomic number (Z) = number of protons
Mass number (A) = protons + neutrons
Neutrons = A – Z
These relationships are used for both atoms and ions, though electrons must be considered separately when charge is involved.
Mass Number: The total number of protons and neutrons in the nucleus of an atom.
This framework provides a foundation for identifying particle numbers in ions, where electrons differ from the atomic form.
Determining Electrons in Atoms and Ions
Electrons determine the overall charge of an atom or ion. In neutral atoms, proton and electron numbers are equal. Ions, however, gain or lose electrons to form charged species.
Neutral Atoms
For a neutral atom:
Number of electrons = number of protons
Neutral atoms therefore have no net charge, as the positive charge of protons is balanced by the negative charge of electrons.
Positive Ions (Cations)
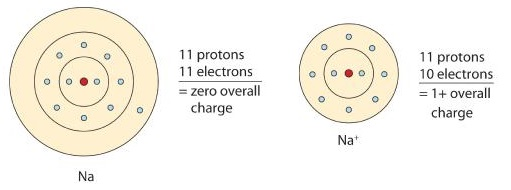
Diagram comparing a neutral sodium atom with a sodium ion (Na⁺), showing that losing one electron decreases the electron count while the number of protons remains constant. This illustrates cation formation relevant to determining subatomic particle numbers. The shell layout uses a simplified Bohr model and includes additional structure beyond OCR requirements. Source
When atoms lose electrons, they form cations. The ionic charge reflects the number of electrons lost.
A positive charge indicates fewer electrons than protons.
Electrons = protons – ionic charge
Negative Ions (Anions)
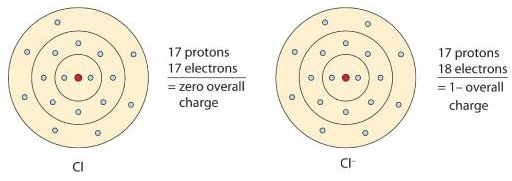
Diagram comparing a neutral chlorine atom with a chloride ion (Cl⁻), showing that gaining one electron increases the electron count while protons remain unchanged. This demonstrates anion formation and supports understanding of electron determination in ions. The simplified Bohr shell structure includes extra detail beyond OCR specification needs. Source
When atoms gain electrons, they form anions. The magnitude of the negative charge indicates the number of electrons gained.
A negative charge indicates more electrons than protons.
Electrons = protons + magnitude of ionic charge
A brief linking sentence helps explain why these relationships matter for chemical formulae and bonding.
Using Atomic Number, Mass Number and Charge
This subsubtopic requires the ability to determine the numbers of protons, neutrons and electrons from the atomic number, mass number and any ionic charge. Students must be able to apply these relationships to both familiar and unfamiliar chemical species.
Steps for Determining Particle Numbers
Use the following structured approach:
Identify the atomic number (Z) to determine the number of protons.
Identify the mass number (A) to calculate the number of neutrons using A – Z.
Determine electron number using the charge:
For a neutral atom, electrons = protons
For a positive ion, subtract the charge from the proton number
For a negative ion, add the magnitude of the charge to the proton number
Why Particle Numbers Matter
Proton number determines element identity.
Neutron number affects isotopic composition and nuclear stability.
Electron number dictates ionic charge and chemical behaviour.
These concepts underpin many later topics in physical, inorganic and organic chemistry, making mastery of particle accounting essential.
Representing Atoms and Ions
Atoms and ions are commonly expressed using standard nuclear notation, pairing atomic number and mass number with chemical symbols. This notation ensures that particle numbers can be derived directly when analysing chemical formulae or equations.
Using Chemical Symbols Correctly
Mass number is written at the top left of the symbol.
Atomic number is written at the bottom left.
Ionic charge is written at the top right if the species is an ion.
This notation provides a concise method for displaying fundamental atomic information needed for determining subatomic particle numbers accurately.
FAQ
An element’s position is determined entirely by its number of protons, known as the atomic number. This defines the element’s identity and location in the periodic table.
Neutrons and electrons do not affect periodic placement. Neutrons only change the isotope of an element, while electrons influence bonding and reactivity but not the element’s classification.
Ease of ion formation depends on how strongly an atom holds its electrons. This is influenced by:
• Nuclear charge
• Electron shielding
• Distance between the nucleus and outer electrons
Atoms with weaker attraction for outer electrons tend to form positive ions more readily, while those with higher attraction tend to gain electrons and form negative ions.
The key factor is how many electrons an atom needs to lose or gain to achieve a stable electron arrangement.
• Metals typically lose electrons because they have few outer electrons and low attraction for them.
• Non-metals generally gain electrons due to higher nuclear charge and stronger attraction for additional electrons.
The balance between nuclear charge, shielding, and outer-shell electron number governs the direction of ion formation.
Some elements can form multiple ions by losing different numbers of electrons. This does not change the number of protons, which remains fixed for the element.
For example, an element forming both 2+ and 3+ ions will have:
• The same proton count
• Different electron counts
• Different chemical behaviour due to altered charge and electron arrangement
These variations directly affect how the ions participate in bonding and reactions.
The number of electrons determines how strongly an ion attracts or repels other charged species. This influences:
• Bond formation
• Reaction pathways
• Interactions with polar molecules
Higher negative charge increases electron repulsion, while higher positive charge increases attraction for electron-rich species. Electron count therefore plays a central role in predicting how ions behave chemically.
Practice Questions
An ion has an atomic number of 16 and a mass number of 32. It has a 2– charge.
Determine the number of protons and electrons in this ion. (2 marks)
• Protons = 16 (1 mark)
• Electrons = 18 (1 mark)
A sample contains ions of two different elements, X and Y.
• Ion X has an atomic number of 12, a mass number of 25, and a 2+ charge.
• Ion Y has an atomic number of 17, a mass number of 37, and a 1– charge.
(a) Determine the number of protons, neutrons, and electrons in ion X.
(b) Determine the number of protons, neutrons, and electrons in ion Y.
(c) Explain why the ions X2+ and Y– have different chemical behaviours despite both having gained or lost electrons.
(5 marks)
(a)
• Protons in X = 12 (1 mark)
• Neutrons in X = 13 (25 – 12) (1 mark)
• Electrons in X = 10 (12 – 2) (1 mark)
(b)
• Protons in Y = 17 (1 mark)
• Neutrons in Y = 20 (37 – 17) (1 mark)
• Electrons in Y = 18 (17 + 1) (1 mark)
(c)
Award up to 2 marks for any two valid points:
• They are different elements because they contain different numbers of protons.
• Their electron configurations differ, even though both have undergone electron gain or loss.
• Different elements have different chemical properties due to differences in nuclear charge and arrangement of electrons.
(Max 5 marks total)

