OCR Specification focus:
‘Use relative molecular mass (Mr) for simple molecules and relative formula mass for giant structures, calculated from relative atomic masses.’
Introduction
Relative mass values allow chemists to compare the masses of substances on a consistent scale. Understanding Mr and relative formula mass underpins stoichiometric calculations across all areas of A-Level Chemistry.
Understanding Relative Mass Concepts
The OCR specification requires students to use relative molecular mass (Mr) for simple covalent molecules and relative formula mass for ionic or giant structures. Both are calculated from relative atomic masses (Ar), which are based on the ¹²C scale, where carbon-12 is assigned an exact mass of 12. These terms form the foundation for later quantitative work involving moles, balanced equations, and yield analysis.
Relative Atomic Mass (Ar) and Its Role
Before exploring Mr and relative formula mass, it is essential to understand the significance of relative atomic mass (Ar). This value represents the weighted mean mass of an element’s isotopes relative to 1/12 of the mass of a carbon-12 atom. Ar values are listed on the Periodic Table and are used directly in calculating Mr and relative formula mass. Ar values account for natural abundance, meaning elements with several isotopes reflect the average mass rather than a whole number.
Relative Molecular Mass (Mr)
The term relative molecular mass (Mr) applies only to simple covalent molecules. These species consist of discrete molecules with identifiable molecular formulae. Mr is determined by adding the Ar values of all atoms present in the molecule.
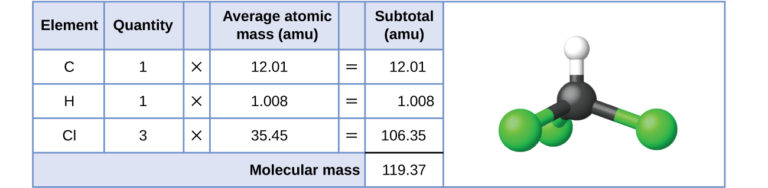
Relative molecular mass of chloroform, CHCl₃, calculated by summing the average atomic masses of C, H and Cl. The table links each element’s Ar to its contribution to the total Mr, while the molecular model reinforces the idea of a simple covalent molecule. Numerical values exceed OCR requirements but clearly illustrate the method. Source
Relative molecular mass (Mr): The sum of the relative atomic masses of all atoms in a simple molecule.
This definition is essential because it distinguishes Mr from relative formula mass, which serves a similar but distinct purpose. Mr has no units because it is a ratio comparing masses.
In covalent substances, Mr reflects the mass of individual molecules, making it especially important in contexts such as molecular formula determination and calculations involving volatile organic compounds. Students must be comfortable identifying when a species should be treated as a molecule in order to apply Mr correctly.
Relative Formula Mass
Some substances do not exist as discrete molecules. Instead, they form giant ionic lattices, giant covalent structures, or other extended arrangements. In these cases, the term relative formula mass is used instead of Mr. Rather than representing the mass of a single molecule, relative formula mass refers to the mass of the empirical formula unit.
Relative formula mass: The sum of the relative atomic masses in the empirical formula of an ionic or giant structure.
This definition is essential because it distinguishes Mr from relative formula mass, which serves a similar but distinct purpose. Mr has no units because it is a ratio comparing masses.
Rather than representing the mass of a single molecule, relative formula mass refers to the mass of the empirical formula unit.
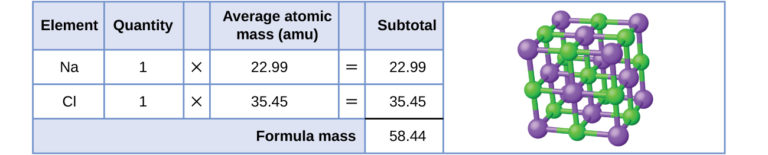
Formula mass of sodium chloride, NaCl, obtained by summing the atomic masses of Na and Cl. The lattice model highlights that NaCl forms a giant ionic structure, justifying the use of relative formula mass rather than Mr. Numerical values exceed OCR requirements but help illustrate the principle. Source
In extended covalent structures such as SiO₂, the giant network makes identifying a discrete molecule impossible; therefore, relative formula mass provides a meaningful measure that aligns with empirical composition.
In metallic structures, the term is rarely used because metals are usually quantified by mass rather than formula unit, but the same principles apply if required.
Importance of Ar Values in Calculations
Because Mr and relative formula mass both rely entirely on Ar values, understanding how Ar is derived from isotopic data is essential elsewhere in the course. However, within this subtopic, Ar should be treated as a provided value used to construct relative masses.
Students must consistently use Periodic Table Ar values, including decimals where necessary, as rounding incorrectly can produce significant errors in larger calculations such as titration analysis or stoichiometric ratios. The OCR specification emphasises the accurate use of Ar in forming Mr and formula mass values.
Applications of Mr and Relative Formula Mass
Both Mr and relative formula mass play a central role in quantitative chemistry. Although detailed calculations are not included here, understanding how these masses are used helps reinforce their importance.
Key Uses of Mr and Relative Formula Mass
Calculating moles using the relationship between mass, molar mass, and amount of substance.
Determining empirical and molecular formulae, where Mr helps identify the true molecular identity from experimental data.
Predicting reacting masses in stoichiometric equations.
Interpreting gas behaviour, where Mr connects to molar gas volumes and the ideal gas equation.
Quantifying volatile substances, particularly relevant for organic compounds.
Analysing hydration, where formula mass is required to determine water of crystallisation.
Choosing Between Mr and Relative Formula Mass
Students must recognise whether a compound is covalent or ionic in order to apply the correct terminology. The decision can be guided by structural knowledge and the nature of bonding.
Use Mr for:
Simple covalent molecules such as oxygen, ethane, or carbon dioxide.
Molecular compounds with discrete structures.
Use relative formula mass for:
Ionic compounds such as MgCl₂ or KNO₃.
Giant covalent solids such as SiO₂.
Any substance without discrete molecules.
The calculation process is identical: sum the Ar values. What differs is the underlying chemical structure.
Structural Implications and OCR Expectations
Understanding when each term applies reinforces broader concepts such as ionic bonding, molecular geometry, and lattice formation. Mastery of terminology ensures clarity when interpreting quantitative questions in later topics, as the OCR specification expects accurate use of chemical vocabulary.
These mass concepts also underpin practical chemistry. For example, preparing standard solutions, comparing yields, and analysing reactions all require accurate mass–mole relationships. Even though students will not perform these calculations here, recognising the role of Mr and relative formula mass provides the conceptual foundation needed for those skills.
Representing Chemical Formulae
Because formulae vary between covalent and ionic species, interpreting chemical formulae correctly ensures that Mr or relative formula mass is calculated accurately.
Empirical formula is crucial for ionic compounds.
Molecular formula is essential for covalent species.
State symbols and context help determine structural type.
These conventions support accurate application of relative mass terminology consistent with OCR expectations.
Final Notes on Terminology
The precise use of Mr and relative formula mass maintains chemical accuracy and aligns with OCR assessment requirements. Distinguishing between molecules and formula units is essential for correct interpretation of chemical quantities and for supporting later quantitative work across the course.
FAQ
Ar values should be used exactly as provided on the data sheet or in the exam. Rounding too early can introduce significant percentage errors.
Where multiple decimal places are given, retain them until the final step of a calculation.
In multi-step problems, students should:
Keep intermediate values unrounded
Round only at the end to the required significant figures
Whole-number Ar values occur when an element is dominated by a single isotope of near-integer mass.
Most elements possess multiple stable isotopes, and Ar reflects their weighted average based on natural abundance.
Therefore, non-integer Ar values are normal and essential for accurate Mr and formula mass calculations.
Not in the same way as simple molecules. Large polymers consist of repeating units rather than discrete molecules with fixed size.
Instead of Mr, chemists refer to the molar mass of a polymer chain or use relative formula mass for the repeating unit.
The true mass varies with chain length, so only the repeat unit mass is typically reported.
Mr values assume average isotopic composition. In reality, molecules can contain different isotope combinations, creating a distribution of possible molecular masses.
Mass spectrometry reveals these patterns as clusters of peaks.
For bulk calculations, average Ar values are still used because they reflect overall natural abundance.
Both types of substance consist of extended networks rather than discrete molecules, so their formulae represent simplest ratios of atoms or ions.
Relative formula mass therefore refers to the mass of one empirical formula unit.
This allows comparison and calculation even though a complete structure contains an effectively infinite number of atoms.
Practice Questions
Chlorine exists as a diatomic molecule, Cl2. Using the relative atomic mass of chlorine (35.5), calculate the relative molecular mass (Mr) of Cl2.
Show your working. (2 marks)
Correct method: Mr = Ar(Cl) × 2 (1 mark)
Correct final answer: 71.0 (1 mark)
A sample of an ionic compound has the empirical formula MgCl2.
(a) Explain why the term relative formula mass is used instead of relative molecular mass for MgCl2. (2 marks)
(b) Calculate the relative formula mass of MgCl2. Use the following relative atomic masses: Mg = 24.3, Cl = 35.5. (3 marks)
(5 marks)
(a)
States that MgCl2 is an ionic compound / forms a giant ionic lattice (1 mark)
States that ionic compounds do not exist as discrete molecules, so Mr cannot be used (1 mark)
(b)
Correct contribution from Mg: 24.3 (1 mark)
Correct contribution from Cl: 35.5 × 2 = 71.0 (1 mark)
Correct total relative formula mass: 95.3 (1 mark)

