OCR Specification focus:
‘Explain relative isotopic mass and relative atomic mass based on the 12C standard; definitions required.’
Understanding relative isotopic mass and relative atomic mass is essential for interpreting atomic structure, comparing different atoms, and applying mass data in chemical calculations across the A-Level Chemistry course.
Relative Isotopic Mass
Relative isotopic mass is a fundamental quantity in chemistry and provides a consistent way to compare the masses of different isotopes. It is based on an agreed international standard that ensures all mass measurements remain comparable across scientific contexts.
Relative isotopic mass: The mass of an atom of a specific isotope compared with one-twelfth of the mass of an atom of carbon-12.
Because this value is defined relative to carbon-12, it has no units and is a pure ratio. Each isotope of an element has its own distinct relative isotopic mass because isotopes differ in the number of neutrons within their nuclei.
Key features of relative isotopic mass
It is always close to a whole number, as it is primarily determined by the number of protons and neutrons.
It is not the same as mass number, though the two may be similar in value.
It is measured experimentally using mass spectrometry, the technique used to determine very small atomic-scale masses.
The choice of carbon-12 as the standard ensures that relative mass measurements remain stable, precise, and internationally comparable.
The atomic mass unit is defined as one-twelfth of the mass of a neutral atom of carbon-12.
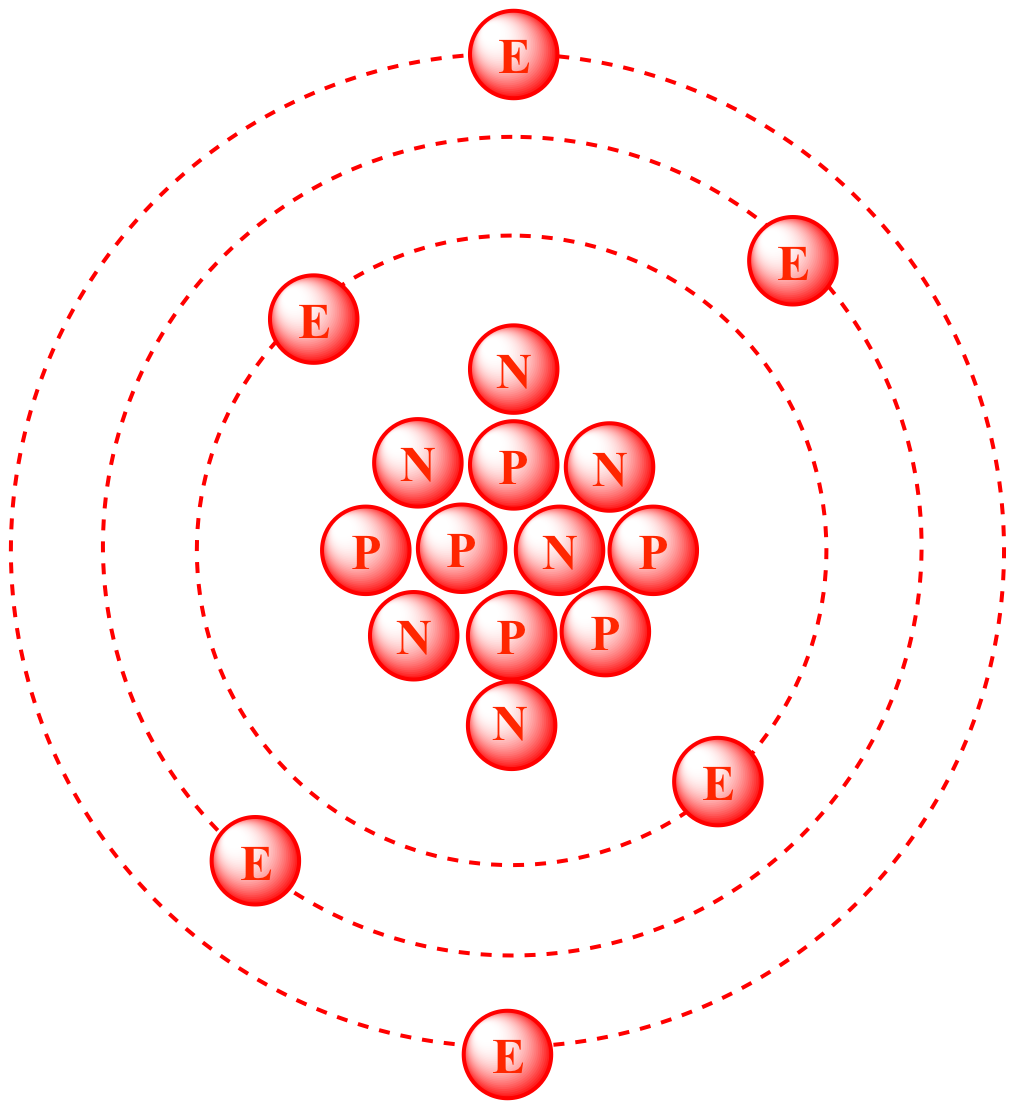
Diagram of a carbon-12 atom showing six protons and six neutrons in the nucleus with electrons surrounding it. This illustrates why carbon-12 is chosen as the reference isotope for defining the relative isotopic mass scale. The image also shows electron positions, which is useful context but not required by this specific syllabus point. Source
Relative Atomic Mass (Ar)
Relative atomic mass is one of the most commonly encountered quantities in chemistry, particularly in calculations involving moles, balanced equations, and periodic trends. It represents the average mass of all naturally occurring isotopes of an element.
Relative atomic mass (Ar): The weighted mean mass of an atom of an element compared with one-twelfth of the mass of an atom of carbon-12.
This quantity accounts for both the relative isotopic masses and their natural abundances, providing a single usable value for each element. Unlike relative isotopic mass, Ar is rarely a whole number because it reflects an average influenced by isotopic composition.
A normal sentence must appear here before including another structured block to maintain clarity and consistency for readers.
Relative Atomic Mass (Ar) = Σ(isotopic mass × isotopic abundance) ÷ 100
Ar = Weighted mean value used in chemical calculations
What influences the value of Ar?
Several factors determine the precise relative atomic mass listed on the periodic table:
Number of naturally occurring isotopes
Relative abundance of each isotope in the environment
Exact isotopic masses determined through mass spectrometry
Geographical variation, since isotopic compositions can differ slightly depending on the source of the element
Because these values are averages, two different samples of the same element may exhibit small variations in measured Ar, but for most elements the differences are negligible in practical chemistry.
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons, so they have different isotopic masses.
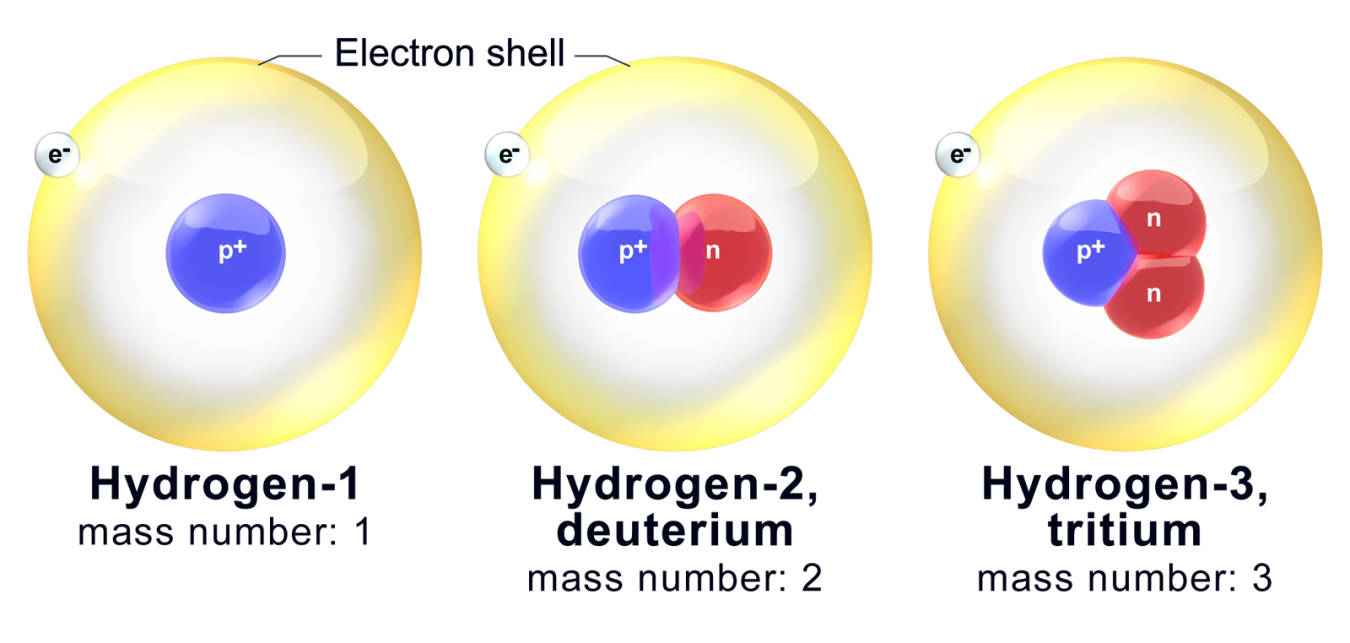
Diagram showing hydrogen and its two naturally occurring isotopes, deuterium and tritium. Each has one proton, but the number of neutrons increases from zero to two, so their isotopic masses increase. The image also names the isotopes explicitly, which goes slightly beyond the OCR specification but clearly illustrates the isotopes concept. Source
The Carbon-12 Standard
The entire system of relative mass measurements hinges on the carbon-12 standard, which assigns the isotope carbon-12 an exact mass of 12.000 atomic mass units. This standardisation allows all atomic masses to be expressed in the same relative scale.
Why carbon-12?
It is a stable, non-radioactive isotope, suitable for accurate comparison.
It is very common in nature, making it a reliable reference.
It creates a convenient and precise scale for mass spectrometry, supporting reproducible measurements across laboratories.
This universal benchmark ensures that both relative isotopic mass and relative atomic mass are based on the same reference point, allowing chemists to compare values consistently.
Distinguishing Key Terms
Understanding the differences between similar mass-related terms is essential for success in A-Level Chemistry. Students must avoid confusing terms that may sound alike but serve distinct purposes.
Important distinctions
Mass number refers to the total number of protons and neutrons in a nucleus and is always a whole number.
Relative isotopic mass, by contrast, is measured relative to carbon-12 and may not be an exact whole number.
Relative atomic mass (Ar) is a weighted mean of isotopic masses and is almost never a whole number.
Chemical problems involving moles or balanced equations nearly always rely on relative atomic masses, whereas discussions of atomic structure or isotopic behaviour require recognition of relative isotopic masses.
The relative atomic mass values printed on the periodic table are these weighted mean values for each element.
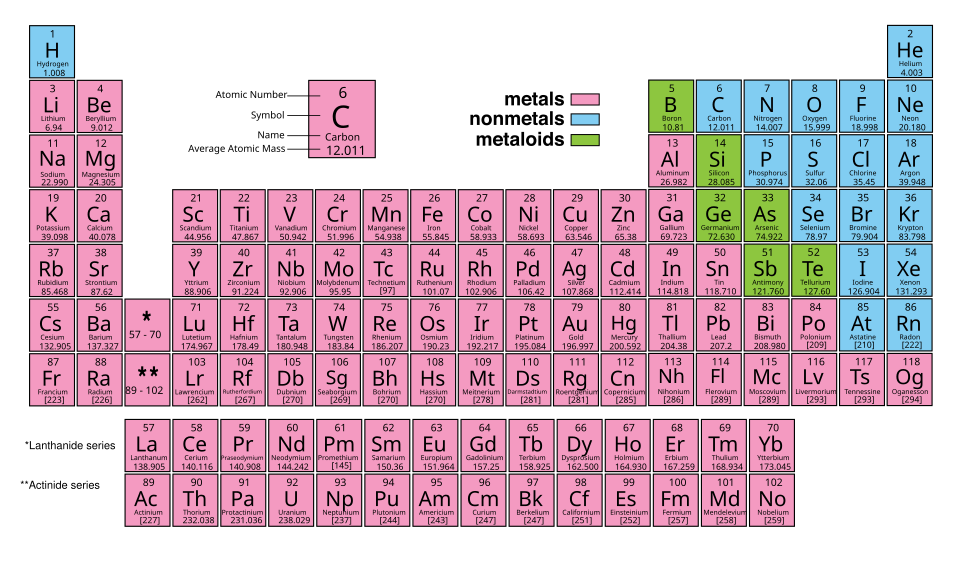
Colour-coded periodic table showing each element’s atomic number, symbol, name, and average atomic mass. Students can see that decimal values, such as chlorine’s 35.5, arise from weighted means of isotopic masses. The colours classify element types, which is extra information not required by this subtopic. Source
Importance of Relative Mass Values in Chemistry
The concepts covered in this subsubtopic underpin many other areas of chemical study. Knowledge of relative masses influences how chemists:
Compare different isotopes and elements
Predict physical properties, such as density and mass-related periodic trends
Perform calculations involving moles, stoichiometry, and reacting masses
Interpret mass spectrometry data used throughout modern chemical analysis
By mastering the definitions and distinctions of relative isotopic mass and relative atomic mass, students build a solid foundation for later quantitative and analytical topics in the A-Level Chemistry course.
FAQ
The mass number is a whole number representing the total number of protons and neutrons in an atom. It is not measured but simply counted.
Relative isotopic mass is experimentally determined using mass spectrometry and may differ slightly from the mass number because it reflects the precise masses of subatomic particles and small mass defects in the nucleus.
Relative atomic mass values can differ due to slight variations in natural isotope abundances in different geographical locations.
Some datasets also update Ar values when new high-precision mass spectrometry measurements become available.
The differences are usually very small, but elements with many isotopes or variable geological sources may show more noticeable variation.
Relative isotopic mass is not exactly equal to the number of nucleons because binding energy affects the total mass of the nucleus.
A greater nuclear binding energy decreases the overall mass of an isotope slightly.
This results in relative isotopic masses that are close to, but not identical to, whole numbers.
Certain elements have highly variable natural isotope compositions, often due to environmental or geological processes.
For these elements, scientific bodies such as IUPAC provide an interval to reflect realistic variations, rather than committing to a single average value.
This approach increases accuracy when the isotopic composition cannot be represented by one meaningful weighted mean.
Elements with many naturally occurring isotopes tend to have relative atomic masses influenced by several abundance-weighted contributions.
This can lead to:
greater sensitivity to small changes in environmental isotope ratios
less stability in the published Ar value
more complex mass spectra with multiple peaks
Elements with one dominant isotope usually have more consistent and reliable relative atomic masses across samples.
Practice Questions
Define the terms relative isotopic mass and relative atomic mass. State clearly the reference standard used for both definitions. (2 marks)
Relative isotopic mass: mass of an atom of an isotope compared with one-twelfth of the mass of a carbon-12 atom. (1 mark)
Relative atomic mass: weighted mean mass of an atom of an element compared with one-twelfth of the mass of a carbon-12 atom. (1 mark) (The reference to carbon-12 must be included for both marks.)
The element neon consists of three naturally occurring isotopes:
20Ne with an abundance of 90.5%
21Ne with an abundance of 0.3%
22Ne with an abundance of 9.2%
(a) Explain why the relative atomic mass of neon is not a whole number. (2 marks)
(b) Describe how a mass spectrometer can be used to determine the relative atomic mass of neon. Your answer should outline the key stages of the process. (3 marks)
(5 marks)
(a)
Relative atomic mass is a weighted mean of all isotopes present. (1 mark)
Natural abundances of isotopes differ, so the average is not a whole number. (1 mark)
(b)
Any three of the following stages, in correct scientific context:
Ionisation: atoms are ionised, usually forming positive ions. (1 mark)
Acceleration: ions are accelerated by an electric field so they all receive the same kinetic energy. (1 mark)
Ion drift or separation: ions are separated according to mass-to-charge ratio, with lighter ions reaching the detector first. (1 mark)
Detection: ions hit the detector, generating a signal proportional to abundance. (credit only once if detection already implied)
(Any three correct stages = 3 marks)

