OCR Specification focus:
‘Isotopes are atoms of the same element with different numbers of neutrons and different masses.’
Introduction
Isotopes provide a foundational understanding of atomic structure by showing how atoms of one element can differ in mass while retaining identical chemical identities and behaviours.
Understanding Atomic Structure
Atoms are composed of three fundamental subatomic particles: protons, neutrons, and electrons. These particles determine an element’s identity, stability, and overall behaviour. The concept of isotopes builds directly on this structural framework, illustrating how variations within the nucleus create measurable differences in mass without changing chemical properties.
Subatomic Particles and Their Roles
Each subatomic particle carries distinct characteristics essential for defining atomic structure.
Protons determine the identity of an element.
Neutrons influence the atom’s mass and nuclear stability.
Electrons govern chemical reactivity and bonding behaviour.
Proton: A positively charged subatomic particle found in the nucleus; it defines the atomic number of an element.
The balance and arrangement of these particles give rise to atomic structure, allowing isotopes to be understood through changes in neutron number rather than changes in proton count.
Neutron: A neutral subatomic particle in the nucleus that contributes to the mass and stability of an atom.
The presence of neutrons stabilises the nucleus by reducing repulsion between positively charged protons. Their number can vary in atoms of the same element, resulting in isotopes.
A separate subatomic particle influences an atom’s interactions outside the nucleus.
Electron: A negatively charged subatomic particle located in orbitals surrounding the nucleus; it determines an atom’s chemical behaviour.
While electron numbers can change to form ions, isotopes always refer exclusively to variation in neutron number.
What Are Isotopes?
The OCR specification emphasises that isotopes are atoms of the same element with different numbers of neutrons and different masses.
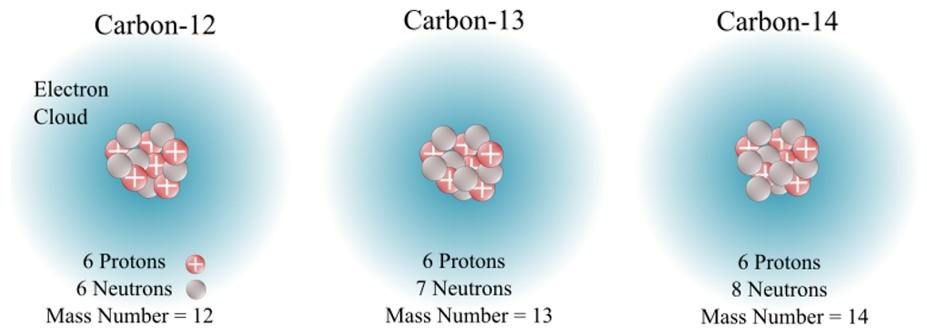
The diagram compares carbon-12, carbon-13 and carbon-14, highlighting their identical proton numbers but differing neutron counts and mass numbers. Source
This means isotopes share the same atomic number but possess different mass numbers, creating subtle physical differences while maintaining identical chemical properties.
Key Characteristics of Isotopes
Same number of protons.
Same electron configuration in neutral atoms.
Different number of neutrons.
Different mass numbers.
Identical chemical properties because chemical behaviour depends primarily on electron arrangement.
Isotope: Atoms of the same element that contain the same number of protons but different numbers of neutrons, resulting in different masses.
Isotopes are frequently represented using standard nuclear notation, where the mass number and atomic number are positioned around the chemical symbol. A normal sentence must follow to ensure clarity. Isotopic notation supports comparison between isotopes of the same element by making proton and neutron numbers easily identifiable.
Importance of Isotopes in Chemistry
Isotopes underpin key concepts across physical and analytical chemistry, including relative atomic mass calculations, nuclear stability, and mass spectrometry. Understanding isotopes also helps explain natural abundance patterns and radioactive decay processes.
Atomic Number, Mass Number and Isotopic Variation
Atomic number (Z) and mass number (A) allow isotopes to be formally described and compared.
Atomic Number (Z): The number of protons in the nucleus of an atom; it defines the element.
The atomic number remains constant for all isotopes of a given element, highlighting why isotopes always belong to the same element. Variation arises from changes in the mass number.
Mass Number (A): The total number of protons and neutrons in an atom’s nucleus.
When neutron number is altered, the mass number changes accordingly, producing a different isotope. The mass difference creates measurable effects in physical properties such as density and rates of diffusion, while leaving chemical reactivity virtually unchanged.
A sentence is included here to ensure appropriate separation from definition blocks. These differences in mass are detectable using analytical techniques, underscoring the importance of isotopes in experimental chemistry.
Neutron Number and Nuclear Stability
The neutron–proton ratio influences the stability of the nucleus. Too few or too many neutrons can result in unstable isotopes, some of which undergo radioactive decay. Although radioactive isotopes fall beyond the scope of the basic definition in this subsubtopic, recognising neutron number as a stability factor helps contextualise isotopic behaviour.
Chemical and Physical Behaviour of Isotopes
Isotopes possess identical electronic structures, meaning they undergo the same reactions, form the same compounds, and display identical qualitative chemical behaviour. This similarity makes isotopic substitution a valuable tool in mechanistic studies and tracer experiments.
In contrast, isotopes exhibit measurable differences in physical properties because mass affects certain behaviours. These variations can include:
Boiling and melting points—often very slight but experimentally measurable.
Density, which increases with additional neutrons.
Diffusion rates, where lighter isotopes diffuse faster.
Vibrational frequencies in covalent bonds, significant in spectroscopy.
Chemical reactions remain consistent across isotopes because electron distribution governs reactivity, not nuclear composition.
Natural Abundance of Isotopes
Most elements exist as mixtures of isotopes in the natural environment. The relative proportion of each isotope is known as its isotopic abundance, influencing the element’s average atomic mass listed in the periodic table. Although detailed calculations belong to later subsubtopics, understanding that isotopic abundance exists supports the conceptual foundation of relative atomic mass.
Examples of Common Isotopic Patterns
Elements such as chlorine and bromine have two or more abundant isotopes that significantly influence their average atomic masses.
Some elements, like fluorine, possess only one stable isotope, simplifying their mass profiles.
Natural isotopic distributions reflect both nuclear stability and historical formation processes.
Why This Subsubtopic Matters
A strong understanding of isotopes and atomic structure basics is essential for progressing through atomic mass calculations, mass spectrometry interpretation, stoichiometric analysis, and later units on bonding and physical chemistry.
FAQ
Isotopes affect measurements because instruments often detect mass rather than identity. Small mass differences between isotopes can shift values such as density or time-of-flight in analytical instruments.
Modern analytical methods account for isotopic variation by calibrating using known isotopic abundances or using mass-sensitive detectors capable of distinguishing isotopes precisely.
The number of stable isotopes depends on nuclear stability, which is determined by achieving a balanced ratio of protons to neutrons.
Elements with very small or very large atomic numbers often struggle to maintain stable nuclei, leading to fewer or no stable isotopes.
Although isotopes behave identically chemically, heavier isotopes move more slowly and form bonds that vibrate at lower frequencies.
This allows chemists to use isotopic labelling to track atoms through a reaction or observe kinetic isotope effects, where rate changes reveal which bonds break or form during the mechanism.
Isotopes with more neutrons have greater mass, which leads to lower vibrational and rotational energy at a given temperature.
This causes molecules containing heavier isotopes to require slightly more energy to change state, resulting in marginally higher melting and boiling points.
Isotopic compositions vary due to processes such as evaporation, biological activity and diffusion, all of which can favour lighter or heavier isotopes.
For example:
Evaporation enriches vapour in lighter isotopes.
Biological systems often prefer lighter isotopes because they react more rapidly.
Geological processes can concentrate heavier isotopes in solid phases.
Practice Questions
Isotopes of an element have different physical properties but similar chemical properties.
Explain why isotopes of the same element have:
a) similar chemical properties
b) different physical properties.
(2 marks)
a) Similar chemical properties
1 mark: Because isotopes have the same number of electrons / same electron configuration.
1 mark: Because chemical reactions depend on electron arrangement, not neutron number.
b) Different physical properties
1 mark: Because isotopes have different numbers of neutrons and therefore different masses. (Any two correct points total 2 marks.)
Carbon has three naturally occurring isotopes: carbon-12, carbon-13 and carbon-14.
Using your knowledge of atomic structure and isotopes, answer the following:
a) State what is meant by the term isotope.
b) Explain why all three carbon isotopes are considered to be the same element.
c) Describe two physical differences you would expect between these isotopes.
d) Explain why carbon-14 is radioactive while carbon-12 and carbon-13 are not.
(5 marks)
a) Definition of isotope
1 mark: Atoms of the same element with the same number of protons but different numbers of neutrons.
b) Why they are the same element
1 mark: All carbon isotopes have 6 protons / the same atomic number.
c) Physical differences
Award up to 2 marks:
1 mark: Different masses / mass numbers.
1 mark: Differences in density, rate of diffusion, or vibrational frequencies (any one).
d) Why carbon-14 is radioactive
Award up to 2 marks:
1 mark: Because carbon-14 has an unstable nucleus.
1 mark: Due to an imbalance in the neutron-to-proton ratio causing nuclear decay.

