OCR Specification focus:
‘Recall names and formulae of NO3–, CO3²–, SO4²–, OH–, NH4+, Zn2+ and Ag+. ‘Nitrate’ and ‘sulfate’ mean NO3– and SO4²–.’
Introduction
Polyatomic ions are essential building blocks in many chemical compounds, and recognising their names, charges, and formulae is crucial for writing accurate chemical equations and ionic compounds.
Understanding Common Polyatomic Ions
Polyatomic ions are groups of atoms bonded together that carry an overall charge. They behave as single species in chemical reactions and must be memorised thoroughly for A-Level success. The OCR specification requires secure recall of several core polyatomic ions, many of which appear frequently in equations, titration contexts, and ionic compound formulae.
Importance of Memorising Polyatomic Ions
Knowing these ions allows you to predict compound formulae, balance equations correctly, and recognise species involved in acid–base, redox, and precipitation reactions. As these ions appear repeatedly across multiple topics, mastery here builds confidence across the course.
Nitrate, Carbonate and Sulfate Ions
These three polyatomic ions appear frequently in inorganic chemistry, especially when forming salts with metals and ammonium.
The Nitrate Ion
The nitrate ion, required by OCR A-Level Chemistry, is an anion commonly found in fertilisers, metal nitrate salts, and acid–base reactions.
This ball-and-stick model shows the nitrate ion with one nitrogen atom and three oxygen atoms arranged in a trigonal planar structure. It highlights nitrate as a single charged polyatomic species. The bond geometry displayed exceeds syllabus requirements but provides useful visual context. Source
Nitrate ion (NO3–): A polyatomic anion consisting of one nitrogen atom and three oxygen atoms, carrying an overall –1 charge.
Metal nitrates are typically soluble, making nitrate salts frequent components in ionic equations. This solubility also means they often act as counter-ions in titrations.
A brief comparison helps clarify terminology: the specification notes that ‘nitrate’ means NO3–, distinguishing it from nitrite, which is NO2–.
The Carbonate Ion
The carbonate ion is a key species in reactions producing carbon dioxide gas.
Carbonate ion (CO3²–): A polyatomic anion containing one carbon atom bonded to three oxygen atoms, with a –2 overall charge.
Carbonates react with acids to produce CO2, making them particularly important when identifying gas-evolving reactions.
The Sulfate Ion
Sulfates are widely encountered in qualitative analysis and solubility rules.
Sulfate ion (SO4²–): A tetrahedral polyatomic anion consisting of one sulfur atom bonded to four oxygen atoms, carrying a –2 charge.
As the OCR specification states, ‘sulfate’ means SO4²–, not to be confused with sulfite (SO3²–), which has a different structure and oxidation state.
These three ions—nitrate, carbonate, and sulfate—form predictable salts and should be memorised with absolute accuracy.
Hydroxide and Ammonium Ions
These two ions are essential for acid–base chemistry and appear repeatedly in titration calculations and ionic equations.
The Hydroxide Ion
Hydroxide, a central species in alkali chemistry, plays a vital role in neutralisation.
Hydroxide ion (OH–): A diatomic anion composed of one oxygen and one hydrogen atom, carrying a –1 charge.
Hydroxide ions react with hydrogen ions (H+) to form water, forming the basis of neutralisation reactions.
The Ammonium Ion
Ammonium acts as the positively charged counterpart to many polyatomic anions.
Ammonium ion (NH4+): A polyatomic cation formed when ammonia accepts a proton, resulting in a species with an overall +1 charge.
This cation features prominently in compounds such as ammonium nitrate (NH4NO3) and ammonium sulfate ((NH4)2SO4), common in both laboratory and industrial chemistry.
This diagram shows the structure of the ammonium ion with nitrogen bonded to four hydrogens, forming the cation NH4+. It emphasises ammonium as a single charged unit. No additional details beyond bonding and charge are included. Source
Transition Metal Ions in the Specification: Zinc and Silver
Although not polyatomic, Zn2+ and Ag+ are included in this subsubtopic because their charges must be recalled without being given, unlike many other metal ions.
Zinc Ion
Zinc forms stable Zn2+ ions, important in displacement reactions and complex ion chemistry.
Zinc ion (Zn2+): A metal cation with a fixed +2 oxidation state, commonly forming colourless compounds and solutions.
Zinc’s consistent oxidation state makes it predictable when forming ionic compounds such as ZnSO4 and Zn(NO3)2.
Silver Ion
Silver is commonly encountered in precipitation reactions, particularly for halide ion testing.
Silver ion (Ag+): A monovalent metal cation with a +1 charge, known for forming characteristic precipitates with halide ions.
Silver nitrate (AgNO3) is frequently used in qualitative analysis due to the clear visual changes produced when Ag+ reacts with halide ions.
Key Ions List (as required by OCR)
To enhance recall, the ions required by the specification are listed below:
Essential Polyatomic Ions
NO3– — Nitrate
CO3²– — Carbonate
SO4²– — Sulfate
OH– — Hydroxide
NH4+ — Ammonium
Essential Metal Ions
Zn2+ — Zinc
Ag+ — Silver
These should be memorised with both names and formulae, as this knowledge is essential for writing correct ionic formulae and equations.
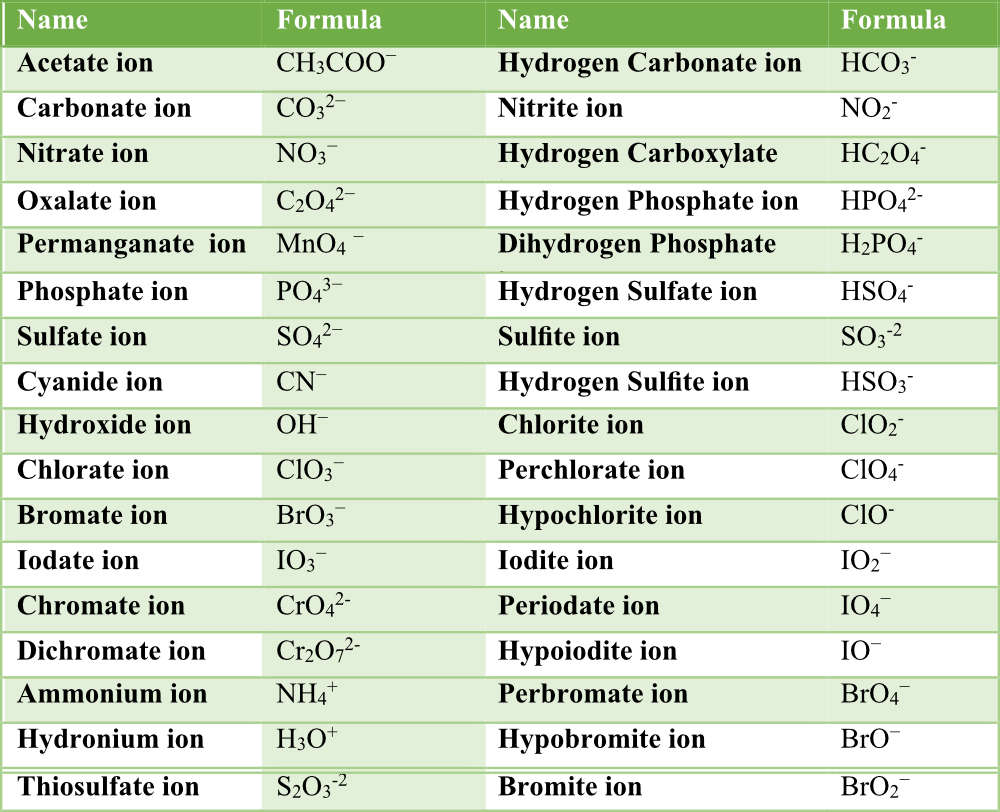
This table summarises common polyatomic ions including nitrate, carbonate, sulfate, hydroxide and ammonium. It reinforces accurate recall of formulas required by the specification. Some additional ions included exceed syllabus requirements but remain helpful for broader context. Source
Strategies for Learning Polyatomic Ions
Students often benefit from structured memorisation approaches. While no single method suits everyone, the following strategies can help form accurate recall:
Create flashcards pairing the ion name with its formula and charge.
Group ions by charge (e.g., –1, –2, +1) to reinforce structural patterns.
Look for patterns in oxygen-containing ions, such as the endings –ate and –ite.
Repeatedly write ionic compounds using these ions to reinforce formula formation skills.
Incorporate these ions into balanced equations to familiarise yourself with their behaviour.
Mastery of these core ions establishes a foundation for more advanced topics involving ionic species throughout the A-Level course.
FAQ
The endings –ate and –ite indicate different numbers of oxygen atoms within related polyatomic ions.
Ions ending in –ate contain more oxygen (e.g., nitrate, NO3–).
Ions ending in –ite contain fewer oxygen atoms (e.g., nitrite, NO2–).
These suffixes help distinguish ions with similar compositions but different oxidation states of the central atom.
Polyatomic ions are typically anions, except for a small number of cations such as ammonium (NH4+).
A useful guideline is:
Ions containing non-metals and oxygen are almost always negatively charged.
Only a few polyatomic cations exist, and ammonium is the only one required for this specification.
The atoms within a polyatomic ion are held together by covalent bonds, forming a stable cluster that carries an overall charge.
When these ions participate in reactions, this charged cluster stays intact because:
Breaking covalent bonds requires far more energy than typical ionic processes.
The ion’s stability allows it to act as a single species when forming ionic compounds or undergoing exchange.
Frequent errors include:
Forgetting to use brackets when more than one polyatomic ion is needed, e.g., writing NH4SO4 instead of (NH4)2SO4.
Confusing similar-looking ions such as sulfate (SO4²–) and sulfite (SO3²–).
Mixing up charges because the ion names appear familiar, such as assuming “nitrate” is NO2–.
Checking both the name and the charge helps avoid these issues.
Although not polyatomic, Zn2+ and Ag+ must be memorised because their charges are not routinely provided in exam questions.
They are included here to ensure students can:
Write correct ionic formulas involving these metal ions.
Balance charges accurately when pairing them with the required polyatomic ions such as nitrate, hydroxide, or sulfate.
Practice Questions
Give the names and charges of the ions with the following formulae:
(a) SO4²–
(b) NH4+
(2 marks)
(a) Sulfate, 2– charge (1 mark)
(b) Ammonium, +1 charge (1 mark)
A student is writing formulae for several ionic compounds but is unsure whether the polyatomic ions they are using have the correct charges.
(a) State the formula and charge of the nitrate ion.
(b) Write the correct formula for the compound formed between ammonium ions and sulfate ions.
(c) Explain why the formula you have written in part (b) is correct, referring to ionic charges.
(d) Name one other polyatomic ion from the OCR-required list and give its formula.
(5 marks)
(a) Nitrate ion: NO3– (1 mark)
(b) (NH4)2SO4 (1 mark)
(c) Explanation referring to charges:
Ammonium ion has a +1 charge (1 mark)
Sulfate ion has a 2– charge (1 mark)
Two ammonium ions are needed to balance the 2– charge on sulfate (1 mark)
(d) Any one of the following for 1 mark:
Carbonate: CO3²–
Hydroxide: OH–
Zinc ion: Zn2+
Silver ion: Ag+

