OCR Specification focus:
‘Predict ionic charge from an element’s position in the periodic table when writing formulae of ionic compounds.’
Introduction
Ions form when atoms gain or lose electrons, and the periodic table provides a reliable guide for predicting the likely ionic charge. Understanding these patterns is essential for writing correct chemical formulae.
Understanding How the Periodic Table Predicts Ionic Charge
The periodic table is structured so that elements in the same group share the same number of outer (valence) electrons. Because chemical behaviour is largely determined by electron configuration, elements within a group tend to form ions with predictable charges.
The Role of Electron Configuration in Ion Formation
Atoms become ions by losing or gaining electrons to achieve a more stable electron arrangement, commonly the electron configuration of a noble gas. Metals typically lose electrons to form positive ions (cations), while non-metals usually gain electrons to form negative ions (anions).
Ion: A charged particle formed when an atom or molecule gains or loses electrons.
The periodic table helps predict this process because the number of electrons lost or gained is linked to the element’s group number.
Predicting Ionic Charge for Main-Group Elements
Students must be able to determine ionic charge efficiently when constructing formulae for ionic compounds. The most reliable patterns come from the s-block and p-block elements.
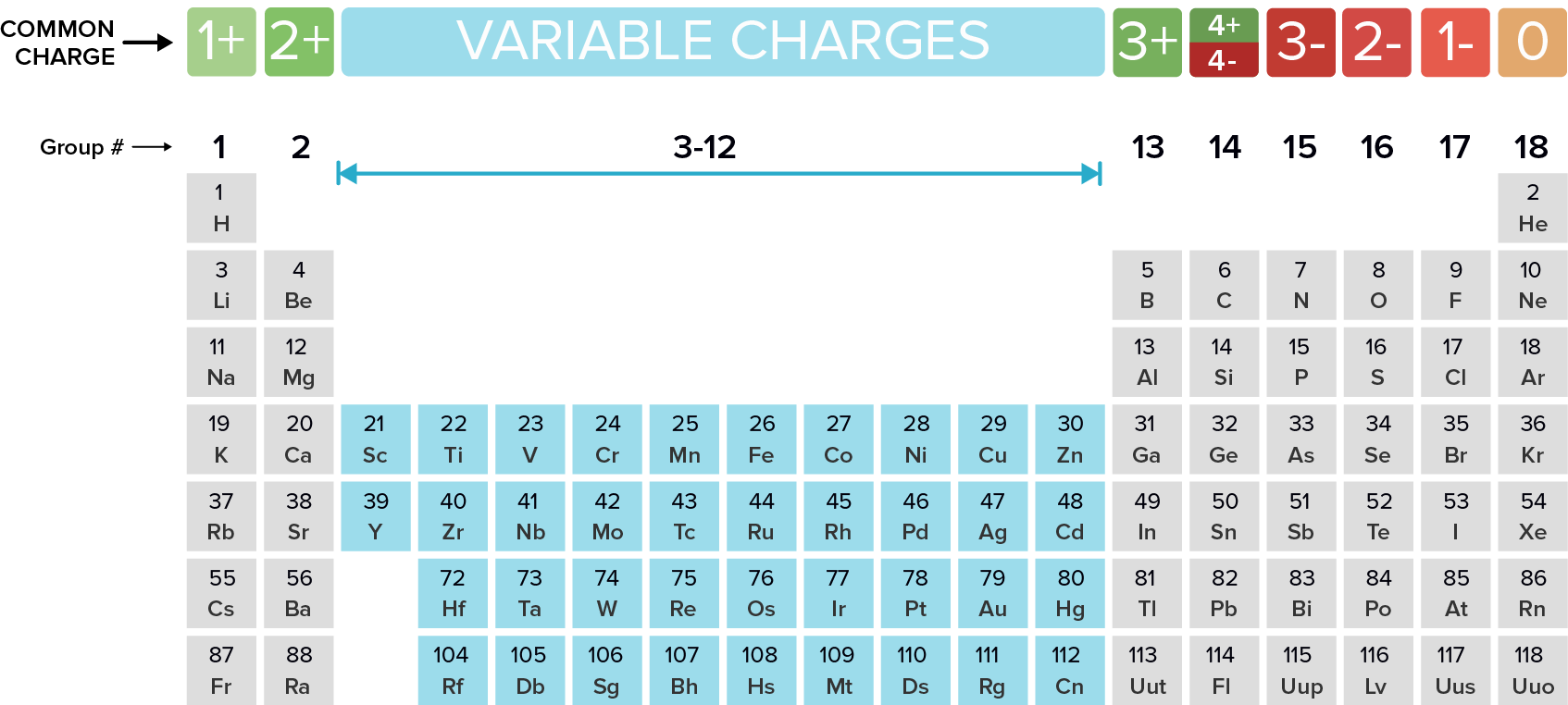
Common ionic charges of main‑group elements, showing how groups 1, 2, 16, and 17 form characteristic ion charges used when predicting ionic species. Source
Group 1: The Alkali Metals
Group 1 metals have one valence electron. They readily lose this electron to achieve a full outer shell.
Form +1 ions
Examples include Li⁺, Na⁺, K⁺
Cation: A positively charged ion formed by electron loss.
These elements are highly reactive, and their predictable +1 charge simplifies formula construction in ionic compounds.
Group 2: The Alkaline Earth Metals
Group 2 metals contain two valence electrons. They lose both electrons to produce:
+2 ions (e.g., Mg²⁺, Ca²⁺, Ba²⁺)
The movement from two valence electrons to a stable configuration is energetically favourable, accounting for the consistency of this pattern across the group.
Groups 3–5: Less Predictable Behaviour
For groups 3 to 5 in the p-block, ion formation is possible but significantly less consistent. These elements may form several different oxidation states, and many form covalent compounds instead of simple ions.
Group 3: Commonly +3, though not always ionic
Group 4: Variable (e.g., +4 or +2), often covalent
Group 5: Can form –3 ions as non-metals, though covalent bonding dominates
While patterns exist, OCR does not require memorisation of variable charges outside the commonly encountered ions.
Group 6: Oxygen Family
Group 6 non-metals have six valence electrons and tend to gain two electrons to complete their outer shell.
Form –2 ions such as O²⁻ and S²⁻
These ions are fundamental in many ionic compounds, including metal oxides and metal sulfides.
Group 7: The Halogens
Group 7 (17) non-metals need one additional electron for a complete outer shell.
Form –1 ions including F⁻, Cl⁻, Br⁻, I⁻
Anion: A negatively charged ion formed by electron gain.
Halide ions appear frequently in reactions, making their predictable –1 charge especially important.
Transition Metals and Variable Charges
Transition metals (d-block elements) do not follow straightforward patterns like the main-group elements. They frequently display variable oxidation states, forming ions with different charges depending on the compound and reaction conditions.
Examples include:
Fe²⁺ and Fe³⁺
Cu⁺ and Cu²⁺
Mn²⁺, Mn⁴⁺, and others
Because of this variability, ionic charges for transition metals must often be given in a question or stated using Roman numerals in names such as iron(II) or iron(III).
Using the Periodic Table to Predict Ionic Charges: Key Patterns
A-Level Chemistry students should internalise the most reliable charge predictions:
Positive Ion Patterns
Group 1 → +1
Group 2 → +2
Group 13 → +3 (not always needed for OCR)
Negative Ion Patterns
Group 15 → –3 (e.g., nitride, phosphide)
Group 16 → –2
Group 17 → –1
Why Periodic Trends Work
The behaviour described above is rooted in the energetic stability gained when atoms achieve full valence shells. The trends relate directly to:
Valence electron number
Nuclear charge
Shielding effects
Ionisation energy and electron affinity patterns across groups
These periodic trends make ion formation predictable for most non-transition elements, fulfilling the OCR requirement to identify ionic charges from periodic table position.
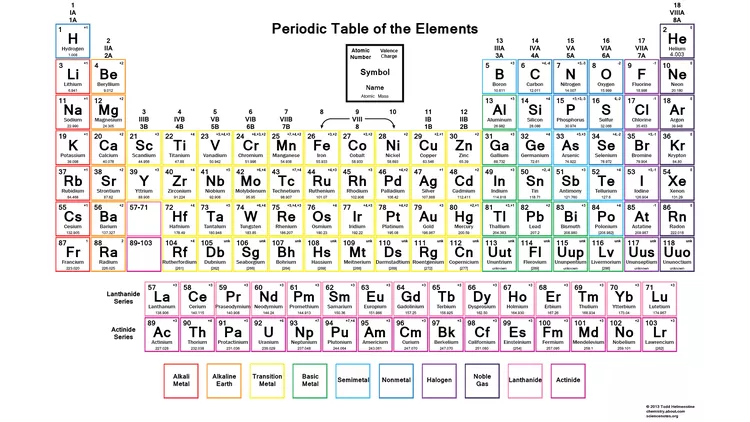
Annotated periodic table showing common ionic charges across the elements, supporting the identification of ions directly from periodic table position. Source
Applying Ionic Charge Predictions When Writing Formulae
When constructing formulas for ionic compounds, charges must balance so that the overall compound is electrically neutral.
Metal cation charge + non-metal anion charge = 0
Charges guide the ratio of ions in a formula
For simple binary ionic compounds:
Magnesium (+2) + chlorine (–1) → MgCl₂
Aluminium (+3) + oxygen (–2) → Al₂O₃
Although worked examples are excluded here, understanding the charge-balancing principle is crucial for determining correct formulae.
Bullet-Point Summary of Charge Predictions
Group 1 metals → +1
Group 2 metals → +2
Group 6 non-metals → –2
Group 7 non-metals → –1
Transition metals → variable charges
Charge balancing ensures neutrality in ionic compound formulae
These structured rules allow students to interpret ionic charge quickly using only the periodic table, as required by OCR A-Level Chemistry.
FAQ
A larger atomic radius means outer electrons experience weaker attraction to the nucleus, making electron loss easier. This is why metals low in the periodic table form positive ions readily.
Smaller non-metal atoms exert stronger attraction on incoming electrons, making electron gain and formation of negative ions more favourable.
Noble gases have full outer electron shells, giving them very high stability and almost no tendency to gain or lose electrons.
Their position provides a reference point: elements form ions that move them towards this stable configuration, helping students predict ionic charges for neighbouring groups.
Elements within a group share the same number of valence electrons, even though these are located in higher energy shells down the group.
This consistent valence electron pattern determines the charge of the ion formed, meaning ionic charge remains constant even as the atom becomes larger.
Group 13 elements can form +3 ions but may also form compounds where covalent bonding is preferred due to higher ionisation energies.
As the energy required to remove three electrons becomes significant for heavier elements, their chemistry becomes less dominated by simple ionic behaviour.
Increased shielding from inner electron shells reduces the attraction between the nucleus and outer electrons.
In metals with significant shielding, electron loss becomes easier, supporting formation of cations.
In non-metals with weaker shielding, electron gain is energetically favourable, promoting formation of anions.
Practice Questions
Sodium and oxygen form an ionic compound.
(a) Predict the charge on a sodium ion.
(b) Predict the charge on an oxide ion.
(2 marks)
(a) Sodium ion has a charge of +1.
1 mark: Correct charge (+1).
(b) Oxide ion has a charge of –2.
1 mark: Correct charge (–2).
Elements in Groups 1, 2, 16 and 17 form ions with characteristic charges.
(a) Explain why elements in Group 1 form +1 ions, referring to electron configuration.
(b) Predict the formula of the ionic compound formed between magnesium and nitrogen.
(c) Explain why transition metals often require Roman numerals in their names when written in chemical formulae.
(5 marks)
(a)
1 mark: States Group 1 elements have one valence electron.
1 mark: Explains they lose this electron to achieve a stable electron configuration/full outer shell.
1 mark: Concludes that this results in a +1 ion.
(b)
1 mark: Recognises magnesium forms Mg2+.
1 mark: Recognises nitrogen forms N3–.
1 mark: Correctly gives formula Mg3N2.
(c)
1 mark: States transition metals can form ions with variable charges/oxidation states.
1 mark: Roman numerals indicate which specific charge is present in a compound.

