OCR Specification focus:
‘Write correct formulae of ionic compounds from ionic charges; other unfamiliar ion charges will be given when required.’
Writing Formulae of Ionic Compounds
Ionic compounds are formed from positive and negative ions combining in ratios that ensure the overall charge is zero. Understanding how to determine the correct formula is essential for accurate chemical communication.
The Nature of Ionic Compounds
Ionic compounds consist of cations (positively charged ions) and anions (negatively charged ions). The chemical formula represents the simplest whole-number ratio of these ions. Students must be able to write correct formulae using given ionic charges or those predicted from the periodic table.
What Ionic Charges Mean
When an atom forms an ion, it either loses or gains electrons, leading to a net electrical charge. This ionic charge is fundamental when establishing the correct ratio of ions in a compound.
Ion: An atom or group of atoms that carries an overall positive or negative charge due to electron loss or gain.
Correctly combining ions requires balancing their charges so the total positive charge equals the total negative charge.
For giant ionic lattices, the formula shows the simplest whole-number ratio of ions, not the total number of ions present in the crystal.
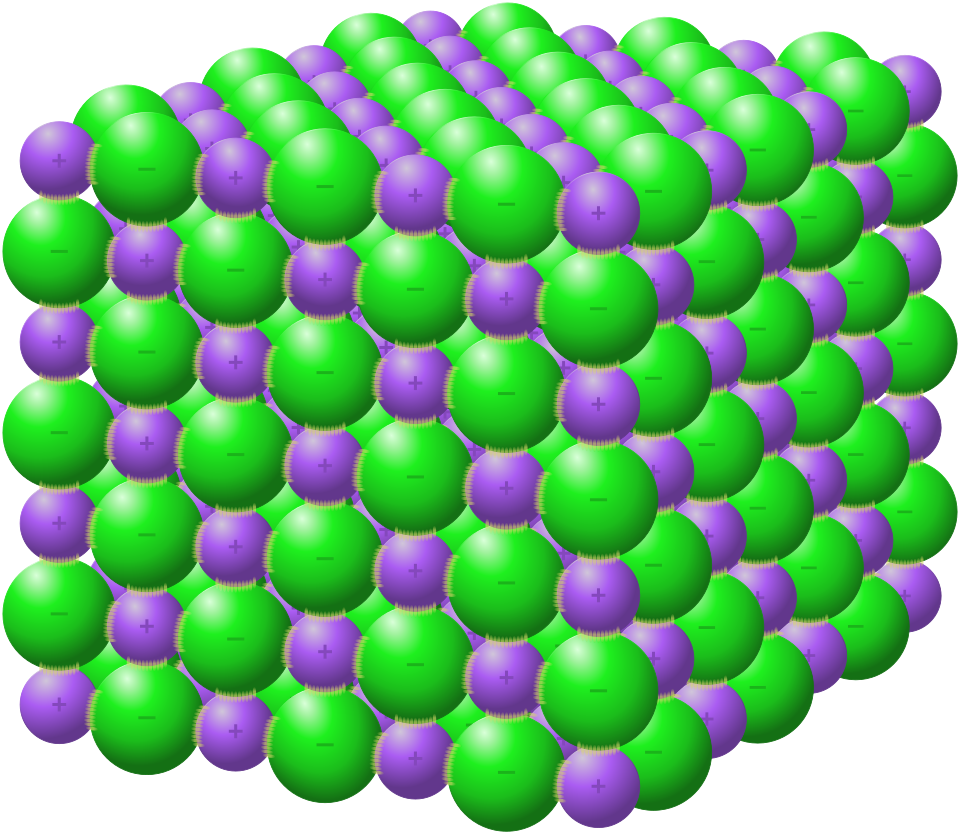
This diagram shows the three-dimensional NaCl lattice, with Na⁺ and Cl⁻ arranged in a repeating 1:1 ratio. It slightly exceeds syllabus detail by illustrating electrostatic interactions but reinforces how the formula represents a simplest ratio. Source
Determining Ionic Charges Before Writing Formulae
Students may be expected to recall or deduce ionic charges. Many charges can be predicted from periodic table groups, whereas unfamiliar ones are supplied when required in examination questions.
Predictable Ionic Charges
Group 1 elements form +1 ions
Group 2 elements form +2 ions
Group 6 elements commonly form –2 ions
Group 7 elements form –1 ions
Other ions, such as those of transition metals, may adopt multiple oxidation states. Where needed, OCR provides the specific charge to use.
Steps for Writing Correct Ionic Formulae
Constructing formulae is a systematic process based on establishing charge balance. The chemical formula should contain the smallest whole-number ratio of ions that results in zero net charge.
Core Process
Identify the cation and its charge.
Identify the anion and its charge.
Balance the charges so total positive equals total negative.
Use subscripts to show the number of each ion needed.
Avoid including charges in the final formula.
Use brackets only when a polyatomic ion requires more than one copy.
This process ensures precision in representing ionic substances and prevents misinterpretation.
Charge Balancing and the Cross-Over Approach
Balancing charges can be supported by the “cross-over” method, in which the numerical value of each ion’s charge becomes the subscript of the other ion. Although useful, students must still reduce ratios to the simplest whole numbers and omit charges from the final formula.
One common method for finding subscripts is the criss-cross method, where you use the magnitude of each ion’s charge as the subscript of the other ion.
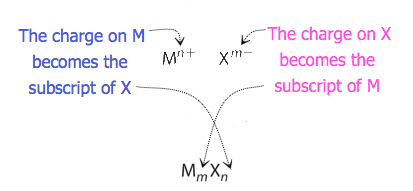
This schematic illustrates the crossing-charges method, where each ion’s charge becomes the partner’s subscript. The general M and X notation goes slightly beyond OCR detail but accurately supports charge-balancing principles. Source
A sentence of explanation ensures clarity: the aim is always to produce an electrically neutral formula, regardless of the technique used.
Polyatomic Ions in Formula Writing
Certain ions consist of more than one atom bonded together and carry an overall charge. These polyatomic ions must be treated as intact units in formula writing.
Polyatomic ion: A charged species containing two or more atoms bonded together, acting as a single ion in chemical reactions.
When you use a polyatomic ion such as sulfate or nitrate, keep the entire group together as one species and only use parentheses when more than one of that ion is needed.
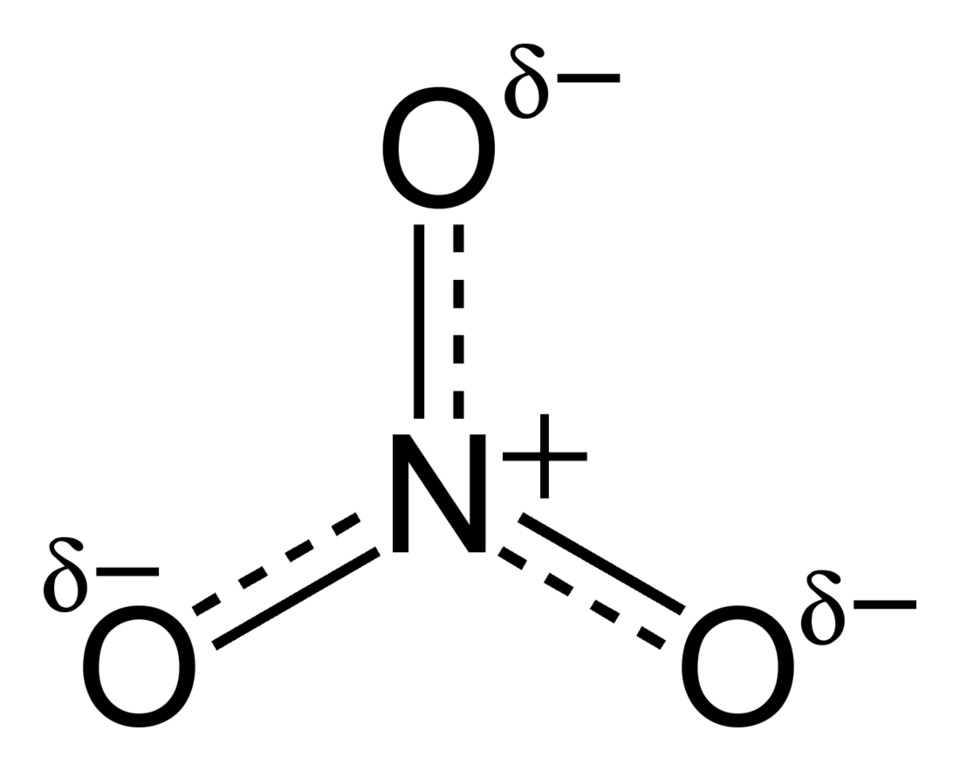
This structure shows the nitrate ion with its overall –1 charge distributed across the oxygen atoms. The depiction of partial charges extends beyond OCR requirements but reinforces the identity of nitrate as a single polyatomic ion. Source
Avoiding Common Errors When Constructing Formulae
Students often make predictable mistakes that can be prevented with consistent checks.
Frequent Pitfalls
Writing the charge in the final formula
Omitting brackets around multiple polyatomic ions
Failing to simplify ratios such as 2:2 to 1:1
Confusing element symbols and their charges
Using incorrect valencies when multiple oxidation states exist
A clear understanding of ionic charge conventions helps avoid errors and strengthens chemical literacy.
Complex Ions and Given Charges
OCR may provide charges for unfamiliar ions such as dichromate, manganate, or transition metal ions with variable oxidation states. Students must incorporate given charges precisely as stated. Accuracy in copying symbols and charges is crucial.
Importance of Precision
A single incorrect letter changes the identity of the ion
Missing a subscript alters the stoichiometric ratio
Misplaced brackets can produce a completely different chemical formula
Ensuring precision reflects the specification requirement to write correct formulae under exam conditions.
Building Confidence with Formula Construction
With practice, writing ionic formulae becomes efficient and reliable. By focusing on charge balance, correct use of brackets, and careful transcription of ion symbols, students produce formulae that accurately represent ionic substances.
Key Concepts to Reinforce
Ionic charges determine the ratio of ions
Total charge must equal zero in the final formula
Brackets are essential when a polyatomic ion appears more than once
Only subscripts, never charges, appear in the final compound formula
Developing confidence in this skill is important for success in later topics such as ionic equations, precipitation reactions, and redox chemistry, all of which rely on accurate chemical formulae.
FAQ
The convention is that the cation (positive ion) is always written first, followed by the anion (negative ion). This ordering reflects how ionic compounds are systematically named.
For example, magnesium chloride is written as MgCl2, never Cl2Mg.
This rule applies even when polyatomic ions are involved.
When ions have equal but opposite charges, the ratio simplifies to 1:1, so no subscripts are required.
Examples include:
Na+ and Cl– form NaCl
Ag+ and NO3– form AgNO3
Even if the cross-over method gives subscripts of 1 and 1, these are omitted.
Ionic formulae represent the empirical formula of the lattice, not the total count of ions present. Simplifying ensures that the written formula reflects the simplest repeating unit.
If you obtain a ratio like Mg2O2, it must be reduced to MgO.
Failure to simplify can produce technically incorrect formulae.
Brackets are essential when a polyatomic ion appears more than once, ensuring the entire ion is treated as a single species.
Process:
Identify the charge of each ion.
Balance charges by finding the lowest whole-number ratio.
Add brackets around the polyatomic ion before adding the subscript outside.
Example: calcium nitrate is Ca(NO3)2.
OCR may introduce ions that students have not memorised to test the ability to apply systematic formula-writing skills rather than recall.
When this occurs:
Use the given charge exactly as written.
Treat unfamiliar ions the same way as familiar ones when balancing charges.
Ensure brackets are used if more than one copy of a polyatomic ion is needed.
This assesses your ability to adapt the method rather than rely on memory.
Practice Questions
Write the correct formula for the ionic compound formed between magnesium ions and nitrate ions.
Explain briefly how you determined this formula. (2 marks)
Mg(NO3)2 (1 mark)
Explanation that magnesium forms Mg2+ and nitrate is NO3–, requiring two nitrate ions to balance the 2+ charge (1 mark)
A compound contains aluminium ions and sulfate ions.
(a) Deduce the correct formula of this compound. (2 marks)
(b) Explain fully why brackets are required in your answer and describe the steps involved in balancing the ionic charges. (3 marks)
(5 marks)
(a)
Al2(SO4)3 (1 mark)
Correct reasoning based on Al3+ and SO4 2– charges balancing to give a 2:3 ratio (1 mark)
(b)
Any three of the following:
Brackets are needed because sulfate is a polyatomic ion that must stay as a single unit (1 mark)
Aluminium has a 3+ charge and sulfate has a 2– charge, so charges must balance to zero (1 mark)
The lowest whole-number ratio is 2 Al3+ ions to 3 SO4 2– ions (1 mark)
The numerical values of the charges become subscripts in the formula (cross-over method), then simplified if needed (1 mark; only allow once)
(Any three points, max 3 marks)

