OCR Specification focus:
‘Explain qualitatively the difference between strong and weak acids in terms of their relative dissociations in aqueous solution.’
Understanding the distinction between strong and weak acids is essential for explaining acid behaviour, predicting reaction outcomes, and interpreting pH-related data across a wide range of chemical contexts.
Strong and Weak Acids: Core Concept
The OCR specification requires students to explain qualitatively how strong and weak acids differ by their degree of dissociation in aqueous solution. This distinction underpins many observable acid behaviours, from pH values to conductivity and reaction rates.
When placed in water, all acids release H⁺(aq), but they do so to different extents. This extent of dissociation defines whether an acid is considered strong or weak. Because dissociation depends on the inherent stability of the species formed, it is not directly related to the concentration of the acid solution itself; rather, it reflects the fundamental nature of the acid.
Dissociation of Acids
What Dissociation Means
Dissociation refers to the separation of an acid into ions when dissolved in water. The more complete this separation, the stronger the acid is classified to be. Water plays a crucial role as both a solvent and a reactant capable of stabilising the ions produced.
Dissociation: The process by which a substance separates into ions when dissolved in water.
After dissociation occurs, the released H⁺(aq) ions do not exist freely but instead form hydronium ions, H₃O⁺(aq). While this species is important, OCR commonly utilises H⁺(aq) notation for simplicity.
A strong acid demonstrates almost complete dissociation at all typical concentrations, whereas weak acids undergo only partial dissociation because their equilibrium strongly favours the undissociated acid molecules.
Strong Acids
Strong acids release H⁺(aq) nearly completely in aqueous solution. This means that the acid exists predominantly as separated ions. The high degree of dissociation is intrinsic to the acid’s structure and its ability to stabilise the conjugate base.
Common strong acids include:
Hydrochloric acid (HCl)
Nitric acid (HNO₃)
Sulfuric acid (H₂SO₄) (first proton only)
Characteristics of strong acids:
High conductivity due to abundant mobile ions
Lower pH compared with weak acids at the same concentration
Faster reaction rates with bases and reactive metals
Conjugate bases that are typically very weak because dissociation is strongly favoured
Strong Acid: An acid that dissociates almost completely in aqueous solution to release H⁺(aq).
Strong acids effectively behave as full proton donors under normal conditions.
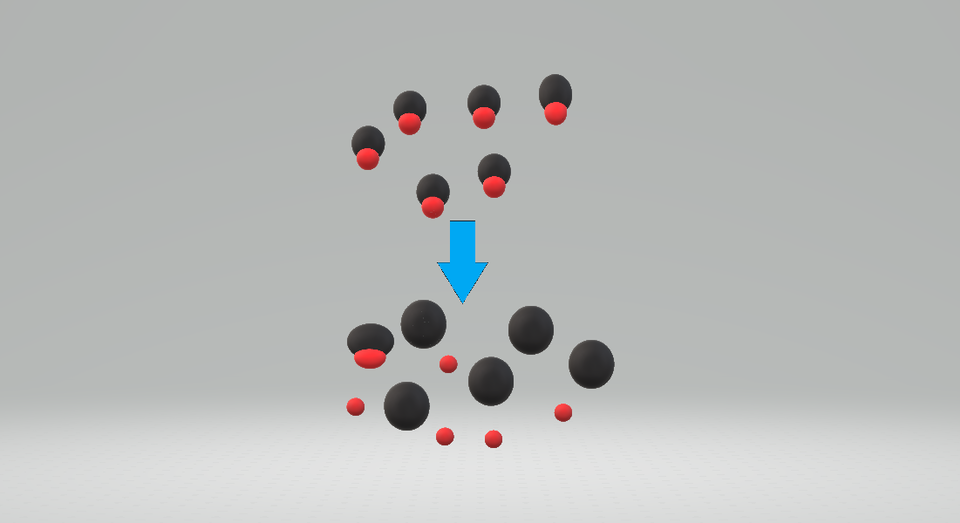
This diagram shows a strong acid in aqueous solution, where almost all particles exist as ions and only a few remain as undissociated molecules. It illustrates how strong acids are almost completely dissociated in water at a given concentration. Students should focus on the high proportion of ions rather than the exact identity of the acid shown. Source
Weak Acids
Weak acids only dissociate partially in aqueous solution, meaning the majority of acid molecules remain undissociated at any given time. Their dissociation establishes an equilibrium between the acid and its ions.
Common weak acids include:
Ethanoic acid (CH₃COOH)
Carbonic acid (H₂CO₃)
Phosphoric acid (H₃PO₄) (stepwise, multiple equilibria)
Characteristics of weak acids:
Significantly higher pH than strong acids at equal concentration
Lower conductivity because fewer ions are present
Conjugate bases that are comparatively stronger
Reactions with bases and metals occur more slowly
Weak Acid: An acid that partially dissociates in aqueous solution, producing an equilibrium mixture of ions and undissociated molecules.
This equilibrium nature is essential for understanding buffer behaviour and acid–base titration curves, which will be explored in later subtopics.
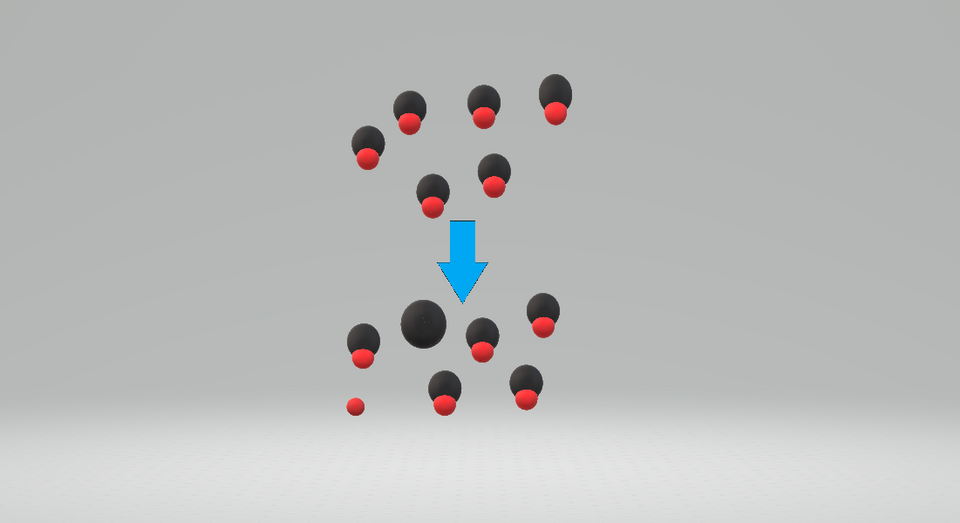
This diagram shows a weak acid in aqueous solution, where most particles remain as undissociated acid molecules and only a small proportion form ions. It illustrates partial dissociation and helps students visualise why weak acids produce a lower concentration of H⁺ at the same concentration. Any additional detail can be treated as contextual rather than examinable.Source
The Role of Equilibrium in Weak Acids
Weak acids exhibit reversible dissociation. The forward reaction produces ions, while the reverse reaction reforms the undissociated acid due to the relatively high stability of the original molecule. This balance is described qualitatively for OCR students, rather than requiring mathematical manipulation.
Key features of weak acid equilibria include:
The position of equilibrium lies far to the left
Only a small proportion of molecules release H⁺(aq)
The equilibrium shifts to restore balance when concentration changes, influencing pH behaviour
Students should appreciate that weak acids do not become strong at higher concentration; their inherent tendency to dissociate remains unchanged.

This figure compares the dissociation equations for a strong acid and a weak acid in water. The strong acid shows a single arrow indicating almost complete dissociation, whereas the weak acid shows a double equilibrium arrow. The use of H₃O⁺ provides additional context beyond the specification but represents the same dissociation process. Source
Comparing Strong and Weak Acids
Although both strong and weak acids release H⁺(aq), their dissociation behaviours lead to distinct chemical properties. OCR expects students to compare them qualitatively, focusing on dissociation rather than numerical values.
Key Contrasts
Extent of dissociation: Strong acids almost complete; weak acids partial, equilibrium established
pH: Strong acids significantly lower pH; weak acids higher pH at equal concentration
Conductivity: Strong acids higher; weak acids lower
Reaction rates: Strong acids react more vigorously; weak acids react slowly
Conjugate base strength: Strong acid → very weak conjugate base; weak acid → stronger conjugate base
These differences emphasise the importance of dissociation as the defining factor.
Factors Affecting Acid Strength
Several structural features influence whether an acid is strong or weak. While OCR does not require detailed mechanistic explanations, students should understand qualitative tendencies.
These factors include:
Bond strength: Weaker H–X bonds enhance dissociation
Stability of the conjugate base: More stable anions favour greater dissociation
Polarity of the H–X bond: Higher polarity promotes ion separation in water
Solvent interactions: Water stabilises ions through hydrogen bonding
Each factor helps determine whether dissociation is complete or partial, shaping the acid’s classification.
Importance in Chemical Reactions
Understanding strong and weak acids is crucial for analysing reaction behaviour. The degree of dissociation determines:
The availability of H⁺(aq)
Reaction vigour and rate
The shape of titration curves
Behaviour in buffer solutions, derived from weak acids
Appreciating these qualitative distinctions allows coherent explanation of acid behaviour across the broader A-Level Chemistry syllabus.
FAQ
Conductivity depends on the number of mobile ions present in solution. Strong acids produce a much higher concentration of ions because they dissociate almost completely.
Weak acids release far fewer ions due to partial dissociation, so their solutions conduct electricity less effectively.
Conductivity comparisons must be made at the same concentration to reflect acid strength rather than dilution effects.
Weak acids exist in equilibrium with their conjugate bases, providing both the proton donor (HA) and proton acceptor (A–) needed for buffering.
Strong acids do not maintain an equilibrium between undissociated acid and conjugate base, as they dissociate almost completely.
A buffer requires a weak acid–conjugate base pair to resist pH change.
Increasing temperature generally favours endothermic dissociation processes, so weak acids may dissociate slightly more at higher temperatures.
However, strong acids remain almost fully dissociated regardless of temperature because their dissociation is already effectively complete.
Changes in temperature do not alter whether an acid is classified as strong or weak.
Strength refers to the extent of dissociation, not how much acid is present.
Increasing the concentration of a weak acid still results in only partial dissociation, while diluting a strong acid does not stop it from dissociating almost completely.
Concentration affects pH but does not alter the inherent dissociation behaviour of the acid.
Conjugate base strength relates directly to acid strength.
Strong acids have very weak conjugate bases because the equilibrium strongly favours dissociation.
Weak acids have stronger conjugate bases because the equilibrium favours the undissociated form.
The stability of the conjugate base—such as its ability to distribute charge—helps determine how readily the original acid donates a proton.
Practice Questions
Explain the difference between a strong acid and a weak acid in terms of their behaviour in aqueous solution. (2 marks)
Strong acids fully or almost completely dissociate in water to release H+ ions. (1)
Weak acids only partially dissociate, forming an equilibrium with a high proportion of undissociated molecules. (1)
Hydrochloric acid and ethanoic acid are both acids but differ greatly in their behaviour in aqueous solution.
(a) Describe what is meant by the term strong acid. (1)
(b) Describe what is meant by the term weak acid. (1)
(c) Explain why a 1.0 mol dm–3 solution of hydrochloric acid has a much lower pH than a 1.0 mol dm–3 solution of ethanoic acid. In your answer, refer to dissociation and the relative concentrations of ions in solution. (3)
(5 marks)
(a) Strong acid: fully or almost completely dissociates in aqueous solution to release H+ ions. (1)
(b) Weak acid: partially dissociates in aqueous solution, producing an equilibrium mixture of ions and undissociated molecules. (1)
(c)
Any three of the following:
Hydrochloric acid produces a much higher concentration of H+ ions in solution because it dissociates almost completely. (1)
Ethanoic acid only partially dissociates, so far fewer H+ ions are released. (1)
Therefore, at the same concentration (1.0 mol dm–3), hydrochloric acid has a much higher concentration of hydrogen ions, leading to a significantly lower pH. (1)

