OCR Specification focus:
‘Describe techniques for preparing a standard solution and carrying out acid–base titrations with appropriate practical precision.’
Introduction
Preparing a standard solution and performing an accurate titration are essential quantitative techniques in A-Level Chemistry, requiring careful measurement, correct apparatus use, and precise procedural steps.
Preparing a Standard Solution
Preparing a standard solution involves accurately dissolving a known mass of solute in a known volume of solution to obtain a solution of precisely known concentration.
Choosing an Appropriate Solute
A suitable solute must be:
Pure and stable, so its mass accurately represents its amount of substance.
Soluble in water, allowing complete dissolution.
High molar mass, reducing percentage weighing errors. Common choices include anhydrous sodium carbonate and potassium hydrogen phthalate.
Equipment Required
Analytical balance (measures mass to 0.001 g precision).
Weighing boat or clock glass.
Beaker for initial dissolving.
Glass rod for stirring.
Volumetric flask, offering precise volume measurement (commonly 100 cm³, 250 cm³, or 500 cm³).
Wash bottle containing deionised water.
Step-by-Step Method for Preparing a Standard Solution
1. Accurately weighing the solute
Weigh a clean, dry weighing boat.
Add the solid and record the new mass.
The mass difference gives the mass of solute.
2. Dissolving the solid
Transfer the solid into a beaker containing a small volume of deionised water.
Use a glass rod to stir until the solute fully dissolves.
Rinse the weighing boat and glass rod into the beaker to ensure all solute is transferred.
3. Transferring solution to the volumetric flask
Pour the solution carefully into the volumetric flask using a funnel.
Rinse the beaker and funnel, adding the washings to the flask.
4. Making up to the calibration line
Gradually add deionised water until approaching the calibration mark.
Add the final drops using a dropping pipette to ensure the bottom of the meniscus sits exactly on the mark at eye level.
5. Inverting to mix thoroughly
Stopper the flask and gently invert at least ten times to ensure a homogeneous solution.
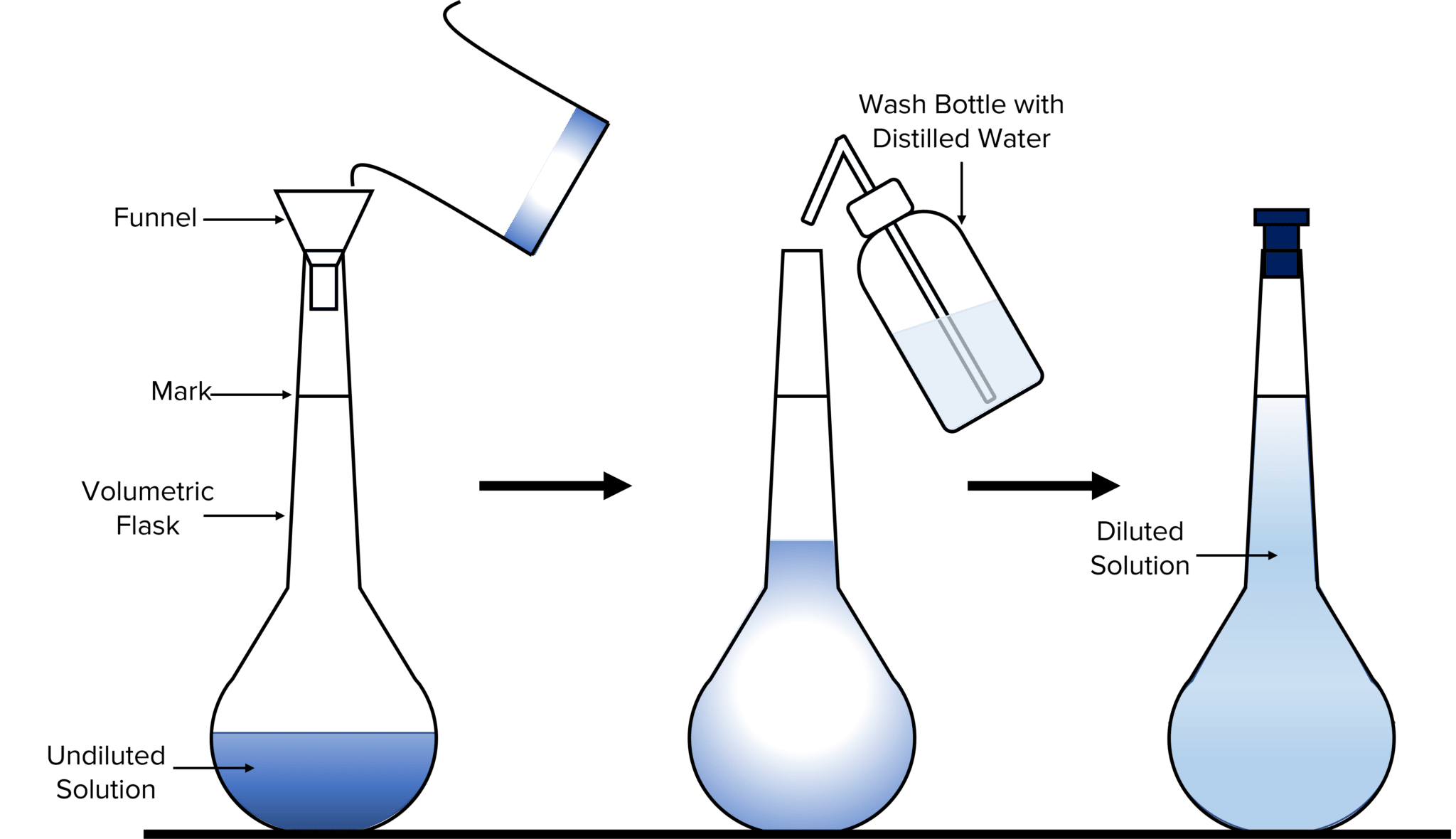
Diagram showing a volumetric flask being filled via a funnel and topped up to the calibration mark using distilled water, followed by inversion to ensure uniform mixing. The diagram also shows a dilution example, which extends slightly beyond the OCR specification but demonstrates identical apparatus and technique. Source
Standard Solution
Standard Solution: A solution of accurately known concentration, prepared using precise mass and volume measurements.
Standard solutions are essential in titrations, enabling reliable and reproducible quantitative analysis.
After preparing a standard solution, a titration may be performed to determine the concentration of another reagent.
Titration Technique
An acid–base titration measures the volume of one reagent required to react exactly with a known volume of another using a suitable indicator to identify the endpoint.
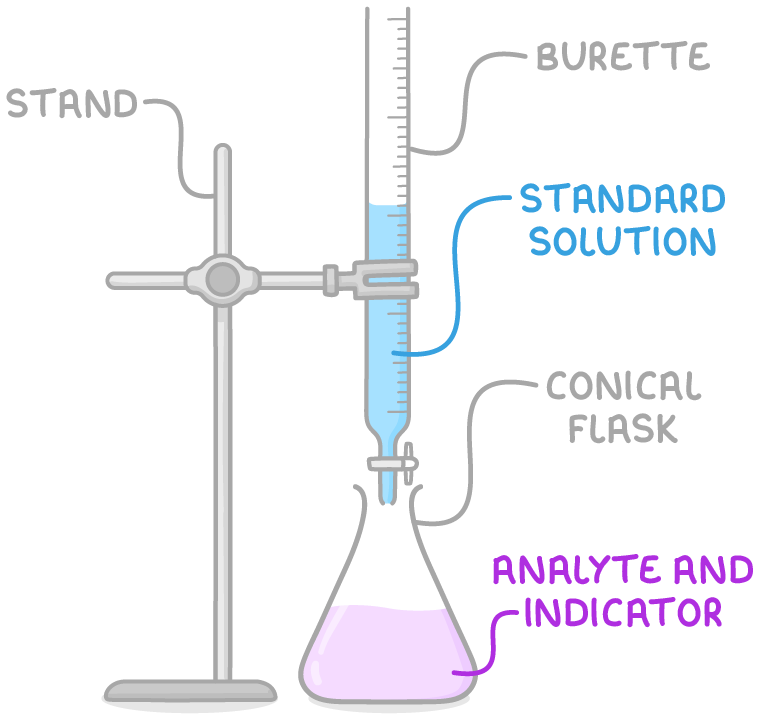
Diagram of an acid–base titration setup with a burette delivering standard solution into a conical flask containing analyte and indicator. Labels reinforce key apparatus terminology expected at OCR A-Level. Source
Required Apparatus
Burette for delivering variable volumes accurately.
Pipette and pipette filler for measuring a fixed volume of solution.
Conical flask for mixing the reactants.
Volumetric solution (standard solution).
Indicator appropriate to the acid–base pair, e.g., methyl orange or phenolphthalein.
Preparing the Burette
Before starting, the burette must be conditioned to prevent dilution errors.
Rinse with deionised water.
Rinse with a small volume of the titrant.
Fill the burette with titrant, ensuring no air bubbles remain in the jet.
Record the initial burette reading to the nearest 0.05 cm³.
Using the Pipette
Rinse the pipette with deionised water.
Rinse with the solution it will deliver (usually the analyte).
Use a pipette filler to draw solution up to the calibration mark.
Release the solution into a clean conical flask.
Endpoint
Endpoint: The point in a titration at which the indicator changes colour, signalling that the reaction is complete.
Indicators must be chosen based on the pH curve of the acid–base combination to ensure a sharp and visible change.
The titration process then proceeds using careful control of reagent addition.
Running the Titration
Add a few drops of the selected indicator to the analyte in the conical flask.
Run the titrant from the burette into the flask while swirling the mixture.
As the colour change approaches, add titrant dropwise to avoid overshooting the endpoint.
Record the final burette reading.
Achieving Precision
Concordant titres (typically within 0.10 cm³ of each other) are required for reliable results.
To ensure precision:
Read burette values at eye level to minimise parallax error.

Photograph showing the concave meniscus in a graduated cylinder, demonstrating the correct point of volume reading at eye level. This reinforces accurate technique for both burette and volumetric flask measurements, despite the page also discussing other liquids beyond OCR requirements. Source
Use white tile beneath the conical flask to improve visibility of the colour change.
Swirl continuously to mix reactants evenly.
Repeat the titration until concordant values are obtained.
Cleaning and Rinsing
Only rinse apparatus with appropriate liquids:
Pipette and volumetric flask: deionised water only.
Burette: deionised water followed by titrant.
Never wash inside the conical flask during the titration sequence, as residual liquid does not affect titre consistency.
Titration
Titration: A quantitative analytical technique in which the volumes of two reacting solutions are measured to determine an unknown concentration.
Titrations, when combined with properly prepared standard solutions, provide accurate stoichiometric data essential for analytical chemistry.
FAQ
A solute is unsuitable if it cannot maintain a constant, known composition. Hydrated salts with variable water content, substances that decompose in air, or solids that readily absorb moisture (hygroscopic) cannot reliably provide an accurate mass.
A solute is also unsuitable if it has very low molar mass, as this increases weighing uncertainty.
Contamination can arise from dirty glassware, airborne dust, or residual chemicals.
To minimise this:
Use thoroughly cleaned and rinsed glassware.
Rinse the volumetric flask with deionised water immediately before use.
Avoid touching the inside surfaces of apparatus.
Ensure the workspace is clear of other reagents or spills.
Failure to invert the flask means the top and bottom regions may have different solute concentrations, producing inconsistent results in titration.
Inverting ensures full homogenisation because simple swirling is insufficient in the narrow-necked volumetric flask.
Parallax error occurs when the burette scale is not viewed directly at eye level, causing readings to appear higher or lower.
Students can reduce it by:
Aligning the eye horizontally with the meniscus.
Using a dark line or stripe behind the burette to make the meniscus edge easier to see.
Ensuring the burette is held completely vertical.
Any water left in the conical flask only dilutes the analyte solution slightly, but this does not change the moles of analyte present, since the entire measured volume from the pipette is still added.
Because only titre differences matter for concordancy, extra water does not alter the final concentration calculation.
Practice Questions
A student prepares a standard solution of sodium carbonate.
Describe how the student should make up the solution to the calibration line once the solid has fully dissolved. (2 marks)
1 mark each for any two of the following:
Add deionised water gradually until close to the calibration mark.
Use a dropping pipette to add water dropwise to bring the bottom of the meniscus exactly to the calibration line.
Ensure the meniscus is read at eye level.
A student performs an acid–base titration to determine the concentration of hydrochloric acid using a standard solution of sodium hydroxide.
Describe the practical steps the student should take to obtain accurate and reliable titre values.
Your answer should include details of apparatus preparation, reading measurements, and how the student ensures precision in the final result. (5 marks)
Award up to 5 marks for the following valid points:
Preparation of apparatus (max 2 marks):
Rinse burette with deionised water, then with the titrant (sodium hydroxide). (1)
Rinse pipette with deionised water, then with the solution it will measure (hydrochloric acid). (1)
Correct use of equipment (max 2 marks):
Fill burette without air bubbles and record initial reading to nearest 0.05 cm³. (1)
Use a pipette and pipette filler to transfer a fixed volume of acid to the conical flask. (1)
Achieving reliable titres (max 2 marks, but total cannot exceed 5):
Add indicator and swirl the flask continuously during titration. (1)
Add titrant dropwise near the endpoint to avoid overshooting. (1)
Precision and concordancy (max 1 mark):
Repeat until concordant titres (typically within 0.10 cm³). (1)
Total marks capped at 5.

