OCR Specification focus:
‘Know formulae of HCl, H2SO4, HNO3, CH3COOH, NaOH, KOH, NH3; acids release H+ (aq), alkalis release OH– (aq).’
Understanding common acids, alkalis and their associated ions underpins all later work in acid–base chemistry, helping to interpret reactions, predict products, and apply fundamental chemical definitions accurately.
Common Acids and Their Characteristics
Acids are central to many chemical processes, and at A-Level it is essential to recognise both their structures and how they behave in aqueous solution. The OCR specification emphasises familiarity with key formulae and the foundational idea that acids are proton donors, meaning they release H⁺(aq) ions when dissolved in water.
Acid: A substance that releases H⁺(aq) ions when dissolved in water.
Acids vary in strength, but all the acids listed here are required knowledge for OCR A-Level Chemistry.
Formulae of Common Acids
The following acids must be memorised exactly as shown:
Hydrochloric acid — HCl
A strong acid that fully dissociates in aqueous solution, widely used in laboratory reactions.
For hydrochloric acid, HCl(aq), the acid dissociates almost completely into H⁺(aq) and Cl⁻(aq) in aqueous solution.
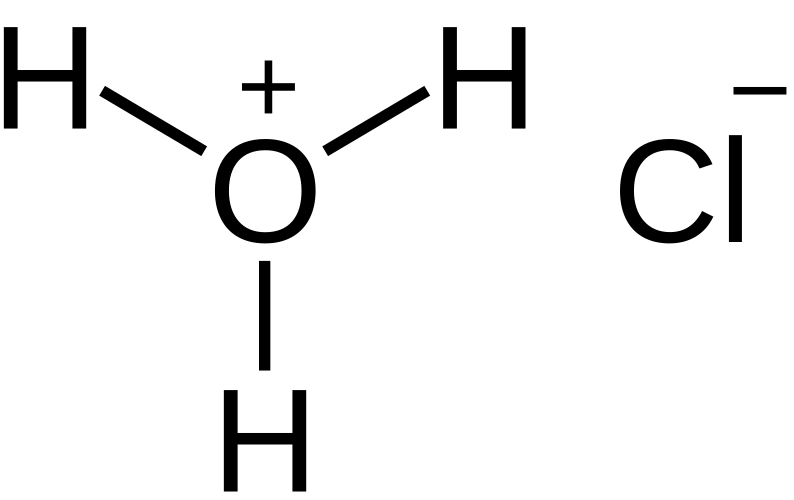
This diagram shows hydrochloric acid dissolved in water, with each HCl molecule transferring a proton to water to form hydronium ions and chloride ions. It emphasises that strong acids in solution behave as collections of solvated ions. Hydronium is shown for completeness, extending beyond the simplified OCR representation of H⁺(aq). Source
Sulfuric acid — H₂SO₄
A diprotic strong acid capable of releasing two protons per molecule in successive steps.
Nitric acid — HNO₃
A strong acid that forms nitrate ions; commonly encountered in redox and acid–base chemistry.
Ethanoic acid — CH₃COOH
A weak acid that partially dissociates, illustrating contrasts in acid strength and ionisation.
Each acid releases hydrogen ions when in aqueous solution, but the degree of dissociation distinguishes strong from weak acids. These molecular differences underpin many observable reaction behaviours.
Alkalis and Hydroxide Ion Formation
Alkalis are a specific type of base that dissolve in water to produce OH⁻(aq) ions. While all alkalis are bases, not all bases are alkalis; for OCR, the focus is on soluble bases that deliver hydroxide ions into solution.
Alkali: A base that dissolves in water to release OH⁻(aq) ions.
This release of hydroxide ions allows alkalis to neutralise acids and form salts through predictable ionic pathways.
Formulae of Common Alkalis
OCR requires knowledge of the following alkalis:
Sodium hydroxide — NaOH
A strong alkali that fully dissociates, widely used in titrations and precipitation reactions.
Potassium hydroxide — KOH
Similar in behaviour to sodium hydroxide, frequently used in analytical chemistry.
Ammonia — NH₃
A weak alkali that forms ammonium ions in solution. It does not contain hydroxide ions initially but produces them through reaction with water.
Normal sentence before definition.
Base: A substance that can accept a proton or neutralise an acid.
Although ammonia does not contain oxygen, it behaves as a base by generating hydroxide ions indirectly via its equilibrium in aqueous solution.
Alkalis are soluble bases; in aqueous solution they release hydroxide ions, OH⁻(aq).
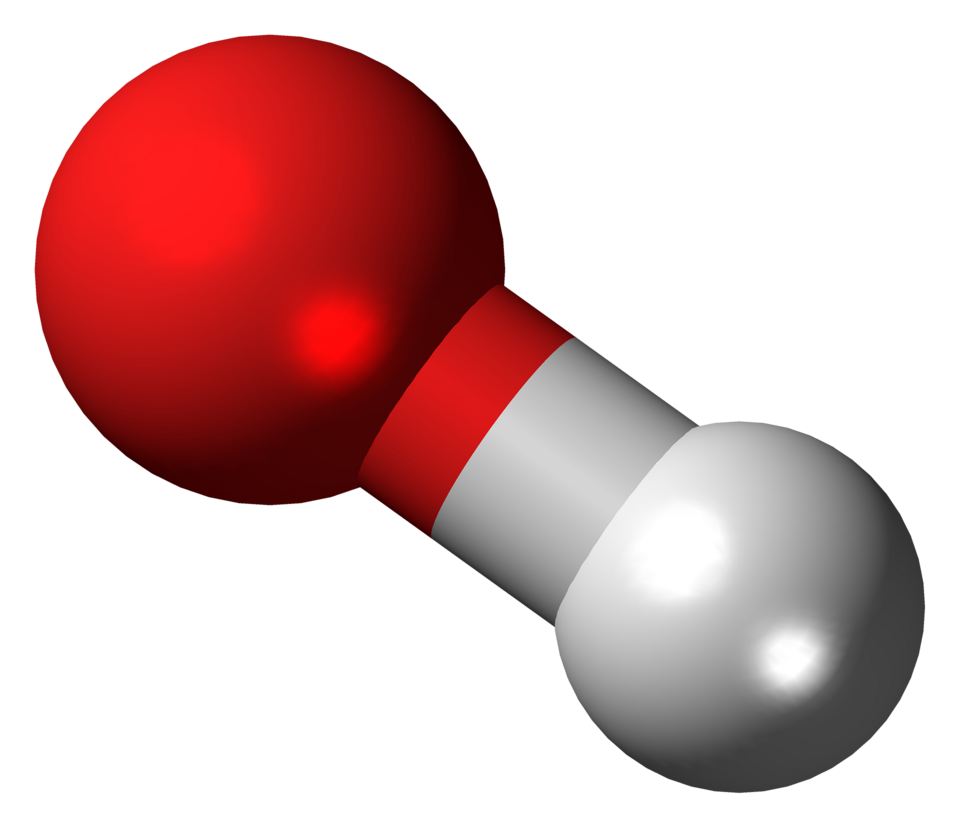
This ball-and-stick model shows the hydroxide ion, OH⁻, highlighting its simple structure as produced by alkalis in solution. It visually reinforces its role in neutralisation reactions. The hosting page also references the hydroxyl radical, which is beyond OCR requirements. Source
Ion Formation and Key Ionic Species
Understanding the ions formed by common acids and alkalis is crucial for writing balanced ionic equations, predicting reaction outcomes, and understanding neutralisation.
Hydrogen Ion
When acids dissolve, they release H⁺(aq), often represented in reactions as being responsible for acidic behaviour. In practice, hydrogen ions exist as hydronium ions (H₃O⁺) in solution, but OCR allows the simplified notation H⁺(aq) for clarity.
Hydroxide Ion
Alkalis supply OH⁻(aq), which reacts with hydrogen ions to form water. This neutralisation process is central to acid–base chemistry and underlies titration methodology.
Ammonium Ion
Ammonia acts differently from metal hydroxides, reacting with water to form the NH₄⁺ ion and hydroxide ions. This dual formation is essential when writing ionic equations for reactions involving ammonia.
Normal sentence before definition.
Ammonium ion (NH₄⁺): A positive ion formed when ammonia accepts a proton in aqueous solution.
When ammonia dissolves in water it accepts a proton to form ammonium ions, NH₄⁺(aq), and hydroxide ions, OH⁻(aq).
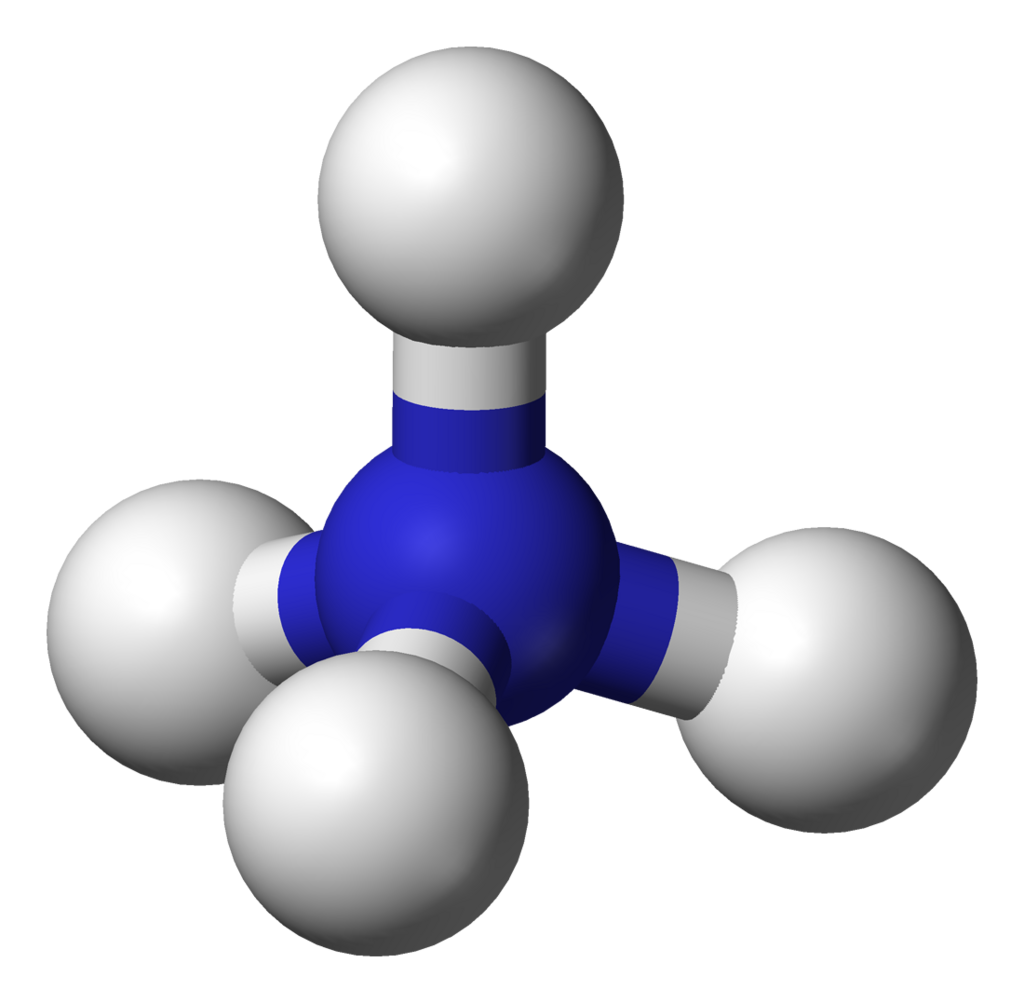
This ball-and-stick model represents the ammonium ion, NH₄⁺, showing its tetrahedral geometry formed when ammonia accepts a proton. It connects structural understanding to the acid–base behaviour discussed in the notes. The hosting page covers broader ammonium chemistry beyond OCR requirements. Source
Neutralisation Reactions and Ion Behaviour
Neutralisation involves the reaction of H⁺(aq) ions with OH⁻(aq) ions to produce water. Understanding this ionic basis allows students to interpret reactions regardless of the acid or alkali involved.
Key Features of Neutralisation
Acid provides H⁺(aq) ions.
Alkali provides OH⁻(aq) ions.
The essential ionic reaction is always:
H⁺(aq) + OH⁻(aq) → H₂O(l)The accompanying ions form the salt.
Neutralisation is not limited to hydroxides; carbonates and oxides can also react with acids. However, this subsubtopic focuses only on the acid–alkali interaction defined by hydroxide and hydrogen ions.
Essential Learning Points for OCR A-Level Chemistry
Required Acid Formulae
HCl (hydrochloric acid)
H₂SO₄ (sulfuric acid)
HNO₃ (nitric acid)
CH₃COOH (ethanoic acid)
Required Alkali Formulae
NaOH (sodium hydroxide)
KOH (potassium hydroxide)
NH₃ (ammonia)
Core Definitions and Concepts
Acids release hydrogen ions in aqueous solution.
Alkalis release hydroxide ions in aqueous solution.
Ammonia forms ammonium ions and hydroxide ions when dissolved.
Neutralisation is fundamentally the reaction between H⁺ and OH⁻.
Key Ionic Species to Recognise
H⁺(aq) – hydrogen ion
OH⁻(aq) – hydroxide ion
NH₄⁺ – ammonium ion
FAQ
Strong acids fully dissociate in water, meaning every acid molecule releases a hydrogen ion. This produces a higher concentration of H+ ions per unit volume.
Weak acids only partially dissociate, so only a fraction of molecules release H+ at any given time.
As a result:
Strong acids generate more H+ ions
Weak acids contain a mixture of dissociated and undissociated molecules
pH is lower for strong acids of the same concentration
Ethanoic acid contains a carboxyl functional group, which allows reversible loss of a proton. The O–H bond in this group is polar but not easily broken compared to strong acids.
Hydrogen bonding and resonance stabilisation within the molecule also reduce the extent of ionisation.
This results in limited H+ release and establishes an equilibrium between CH3COOH and CH3COO− + H+.
A base becomes an alkali only if it dissolves in water to form aqueous ions.
Key points:
All alkalis are bases
Not all bases are alkalis
Solubility is essential: only soluble bases release OH− ions in solution
Examples:
NaOH and KOH are alkalis because they dissolve fully
CuO is a base but not an alkali because it is insoluble
Ammonia is a molecular compound, not an ionic solid. It does not begin with hydroxide ions but generates them through reaction with water.
Its behaviour depends on:
Proton acceptance from water
Formation of NH4+ and OH− ions
Establishing an equilibrium rather than full dissociation
This makes ammonia a weak alkali, unlike metal hydroxides that dissociate completely.
Ions are charge carriers, so conductivity depends on both the number and mobility of ions present.
Acids produce H+ ions; alkalis produce OH− ions. Strong acids and alkalis conduct well because they fully dissociate.
Weak acids and weak alkalis conduct poorly because they produce fewer ions due to limited dissociation.
Practice Questions
Hydrochloric acid and ethanoic acid are both acids found in the OCR A-Level Chemistry specification.
(a) State what is meant by an acid.
(b) Explain why hydrochloric acid is described as a strong acid, whereas ethanoic acid is described as a weak acid.
(2 marks)
(a) 1 mark:
Acid: A substance that releases H+ ions in aqueous solution.
(b) 1 mark:
Hydrochloric acid fully dissociates in water (strong).
Ethanoic acid only partially dissociates (weak).
Ammonia, NH3, is classified as an alkali even though it does not contain the hydroxide ion.
(a) Explain how ammonia forms hydroxide ions when dissolved in water.
(b) Write the ionic equation for the reaction involved.
(c) Describe how the ammonium ion forms and give its formula.
(d) State the difference between a base and an alkali in terms of their behaviour in aqueous solution. (5 marks)
(a) 1 mark:
Ammonia reacts with water to accept a proton and produce hydroxide ions.
(b) 1 mark:
NH3 + H2O → NH4+ + OH−
(c) 2 marks:
Ammonium ion forms when ammonia accepts a proton (1 mark).
Correct formula NH4+ (1 mark).
(d) 1 mark:
A base accepts protons; an alkali is a soluble base that releases OH− ions in aqueous solution.

