OCR Specification focus:
‘Use oxidation numbers to write formulae and include Roman numerals where elements have multiple oxidation states, e.g. iron(II) and iron(III).’
Using Oxidation Numbers in Names and Formulae
Oxidation numbers are essential for identifying oxidation states, naming compounds with metals that form multiple ions, and constructing correct chemical formulae based on electron-transfer principles.
Introduction
Oxidation numbers help chemists describe electron changes, distinguish between different oxidation states of elements, and write systematic names and formulae accurately across a wide range of inorganic compounds.
Understanding Oxidation Numbers
Oxidation numbers are assigned values that indicate the hypothetical charge an atom would have if bonding were completely ionic. These values allow chemists to track redox changes and identify species that can form more than one type of ion.
Basic Principles of Oxidation Number Assignment
When using oxidation numbers in naming and writing formulae, certain rules must be applied consistently. These rules support clarity and prevent ambiguity in chemical communication.
Elements in their uncombined form always have an oxidation number of 0.
Simple ions possess oxidation numbers equal to their ionic charge.
Oxygen typically has an oxidation number of –2, except in peroxides where it is –1.
Hydrogen usually has an oxidation number of +1, except in metal hydrides where it is –1.
The sum of oxidation numbers in a neutral compound is 0, and in a polyatomic ion equals the ion’s overall charge.
These rules provide the foundation for naming compounds that involve variable oxidation states.
Using Oxidation Numbers in Chemical Names
Some elements, particularly transition metals, can form ions with multiple possible oxidation states. To avoid ambiguity, chemists use Roman numerals in names to specify the oxidation number of the metal ion present.
Roman Numerals and Transition Metals
Roman numerals appear in parentheses immediately after the element’s name. They show the oxidation number of the metal, not the charge of the compound as a whole.
Roman numeral notation: A system used in chemical nomenclature to indicate the specific oxidation number of a metal capable of forming multiple ions.
A full chemical name must therefore include the oxidation state whenever a metal has more than one possible value. For example, “iron(II)” identifies Fe²⁺, whereas “iron(III)” identifies Fe³⁺. A single metal may take several oxidation states, and the appropriate numeral ensures the compound’s identity is unambiguous.
After defining oxidation states in names, it becomes possible to understand their role in constructing accurate chemical formulae for ionic compounds.
Common practice is that only metals capable of forming more than one oxidation state use a Roman numeral.
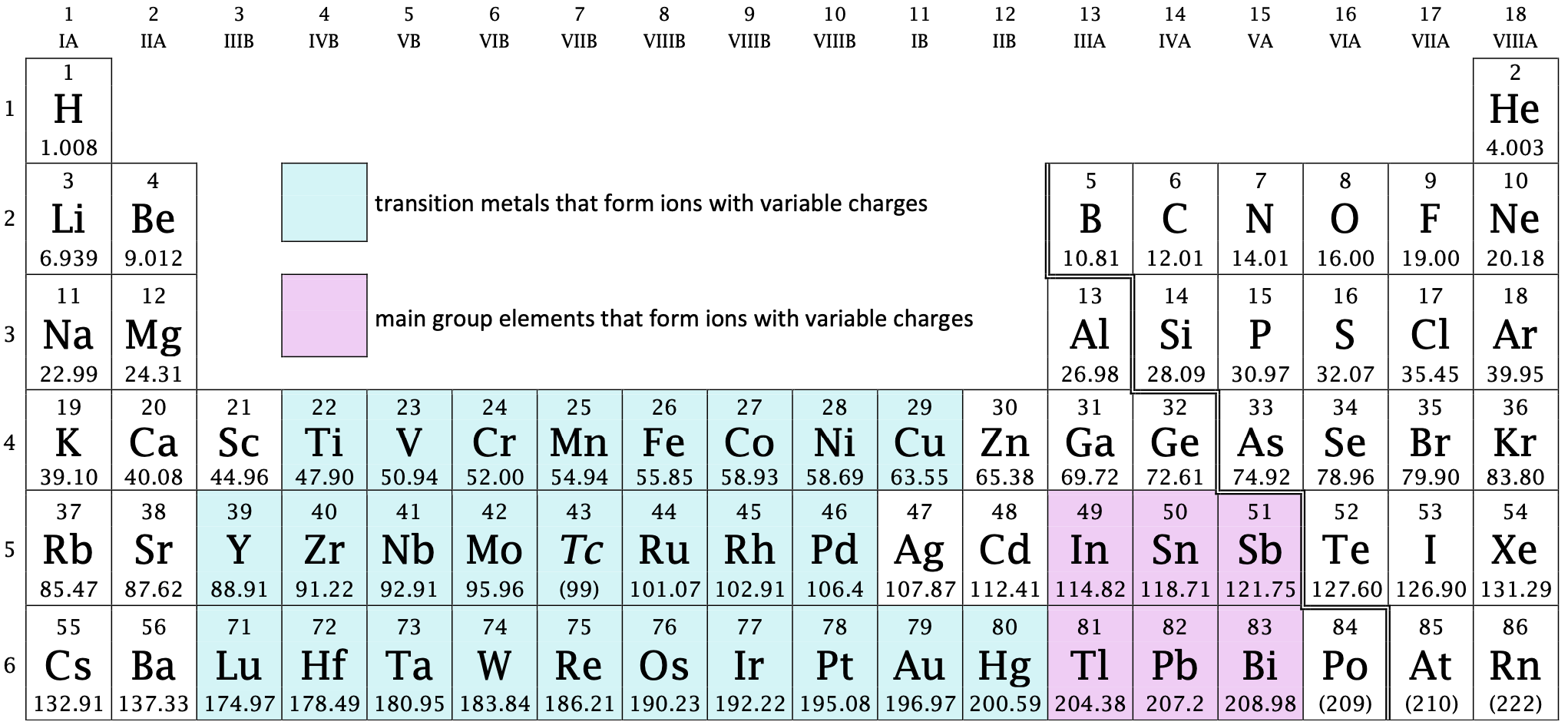
This periodic table highlights metals that commonly form ions with variable oxidation states. Such elements require Roman numerals in compound names to specify their oxidation number. The image includes additional elements beyond OCR requirements but remains fully chemically accurate. Source
Using Oxidation Numbers to Write Formulae
Writing formulae requires the correct balance of positive and negative charges. Oxidation numbers guide this balancing by indicating how many electrons an atom loses or gains.
Steps for Constructing Formulae
Use the following structure when applying oxidation numbers to write chemical formulae:
Identify the relevant oxidation numbers for each element or ion.
Balance the overall charges so that the total positive and negative contributions equal zero.
Use subscripts to show the number of ions or atoms needed to achieve charge balance.
Treat polyatomic ions as intact units unless the formula requires otherwise.
Ensure that the formula is written in its simplest whole-number ratio.
These steps allow students to convert oxidation information into precise symbolic formulae.
A short explanation of the underlying relationship between oxidation number and charge helps reinforce this process.
Oxidation number: A value assigned to an atom in a compound or ion representing its effective charge based on electron gain or loss.
Using oxidation numbers in this way ensures that formulae for compounds containing transition metals, polyatomic ions, or complex ionic species are systematically constructed.
Naming Compounds Using Oxidation Numbers
With formulae established, oxidation numbers also determine the full systematic names of compounds. This naming system follows the rules of the International Union of Pure and Applied Chemistry (IUPAC).
Key Features of Naming
Metal first, then non-metal for ionic compounds.
Roman numerals included for metals that exhibit more than one oxidation state.
Anion names ending in “–ide”, “–ate”, or “–ite” depending on whether the compound contains a simple ion or an oxyanion.
Oxidation numbers applied to determine the correct anion and cation combination before constructing the full name.
This structured approach ensures that names correspond directly to the oxidation states shown in the formula.
A final concept reinforces how oxidation numbers guide the entire nomenclature process.
Variable oxidation state: The ability of an element, usually a transition metal, to exist in more than one oxidation number, leading to multiple possible ionic forms.
Including oxidation numbers explicitly in names avoids confusion when dealing with metals that exhibit this property.
Applying Oxidation Numbers Beyond Basic Compounds
Oxidation numbers also apply to complex ions and coordination compounds, enabling consistent naming and formula-writing across a broad range of chemical species. The same principles of balancing oxidation states to obtain the correct formula still apply, ensuring all compounds remain systematically described.
Summary of Key Uses
Indicating oxidation states in compound names.
Determining correct formulae for ionic species.
Distinguishing between different forms of the same element.
Supporting clarity in written chemical communication.
These uses make oxidation numbers an essential part of A-Level Chemistry nomenclature and formula construction.
These patterns are used alongside known oxidation states to deduce the state of a particular atom in a formula.
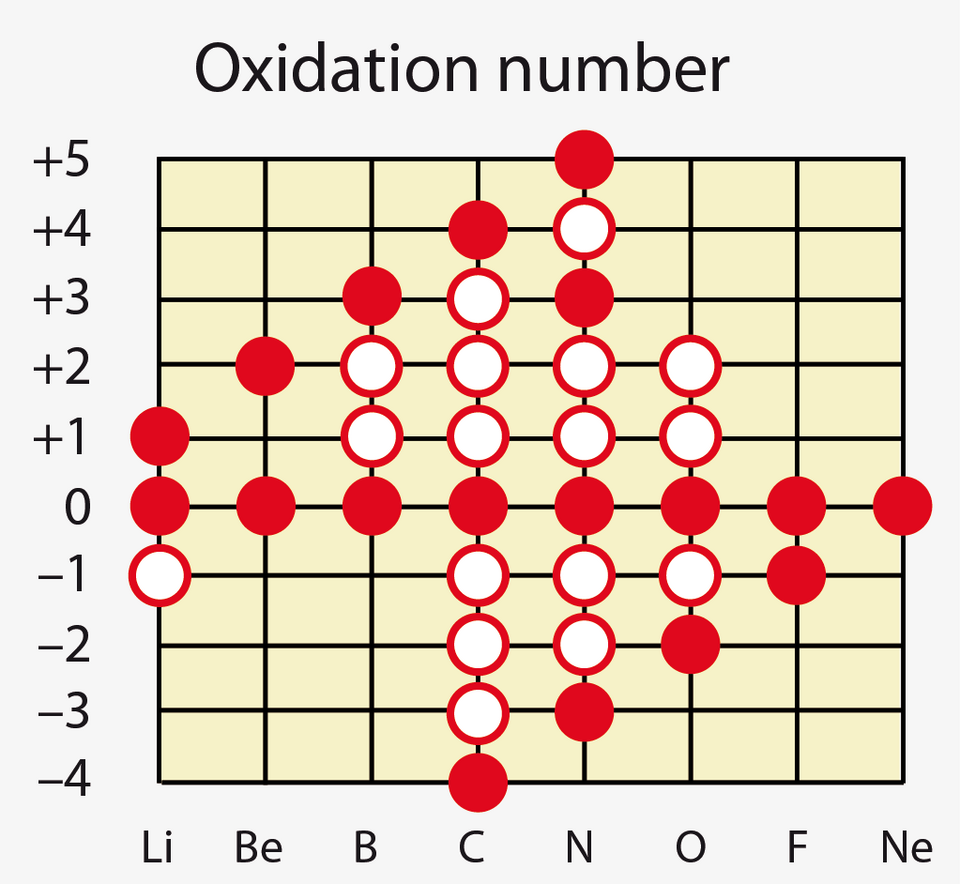
This graph displays common and less common oxidation numbers for Period 2 elements. Filled and open circles indicate the range of states an element may adopt. Some values exceed those emphasised in the OCR course but remain fully valid and help illustrate variable oxidation behaviour. Source
FAQ
Oxidation numbers and ionic charges can sometimes be the same value, but oxidation numbers are a formalism used to track electron distribution, not a direct measurement of charge.
In naming, oxidation numbers identify which oxidation state a metal adopts, even when the compound is not fully ionic. This allows consistent naming across ionic, covalent, and mixed bonding environments.
The number of oxidation states depends on the relative stability of electron configurations formed when d electrons are removed.
Transition metals with similar energies between their ns and (n–1)d orbitals often exhibit several stable oxidation states. Those with larger energy gaps tend to have fewer.
Non-metals typically show a narrow range of oxidation numbers in simple compounds, so ambiguity is less common.
Metals, especially transition metals, can form several cations with different oxidation numbers, and Roman numerals prevent confusion when naming compounds containing them.
Oxidation numbers allow checking whether total positive and negative values are balanced.
A correctly written formula must satisfy:
The sum of oxidation numbers equals zero for a neutral compound.
The sum of oxidation numbers equals the overall charge for a polyatomic ion. If these rules fail, the formula is not valid.
Polyatomic ions have fixed, well-defined oxidation numbers for each atom. When they bind to a metal, their total charge constrains the metal’s oxidation number.
For example:
A metal bonded to nitrate must accommodate the ion’s overall –1 charge.
This allows deduction of the metal’s oxidation number based on charge balance.
This holds true even when multiple polyatomic ions coordinate to a single metal centre.
Practice Questions
Iron can form ions with different oxidation numbers.
(a) State the oxidation number of iron in iron(III) oxide, Fe2O3.
(b) Explain why a Roman numeral is needed in the name of this compound.
(2 marks)
(a) Iron has oxidation number +3 in Fe2O3.
+3 stated for iron (1 mark)
(b) Roman numerals are required because iron can exist in more than one oxidation state.
Must state that iron forms multiple oxidation states (1 mark)
Copper forms compounds with two common oxidation numbers.
(a) Determine the oxidation number of copper in CuO.
(b) Determine the oxidation number of copper in Cu2O.
(c) Explain how oxidation numbers are used to generate the correct systematic names for these compounds and why this notation is necessary.
(5 marks)
(a) Copper in CuO has oxidation number +2.
Correct value +2 (1 mark)
(b) Copper in Cu2O has oxidation number +1.
Correct value +1 (1 mark)
(c)
Names must include Roman numerals to indicate oxidation numbers (e.g., copper(II) oxide, copper(I) oxide). (1 mark)
Explanation that Roman numerals distinguish between different ionic forms of the same element. (1 mark)
Reasoning that this avoids ambiguity in formulas and identifies which oxidation state is present. (1 mark)
(5 marks total)

