OCR Specification focus:
‘Write full equations for redox reactions of metals with acids to form salts and hydrogen; ionic equations are not required here.’
Introduction
Reactions between metals and acids are fundamental redox processes that produce characteristic products. Understanding these reactions helps explain salt formation, hydrogen evolution, and redox behaviour.
Understanding Metal–Acid Reactions
When a metal reacts with an acid, a salt and hydrogen gas are formed. These reactions are essential illustrations of redox chemistry, where electrons are transferred between species. Students must write full equations rather than ionic equations, focusing on accurate product prediction and correct formula construction.
The General Reaction Pattern
Most metals above hydrogen in the reactivity series react with dilute acids. The products formed depend on the acid used and the charge of the metal ion produced.
Typical reaction pattern:
Metal + Acid → Salt + Hydrogen
The metal is oxidised, and the hydrogen ions in the acid are reduced.
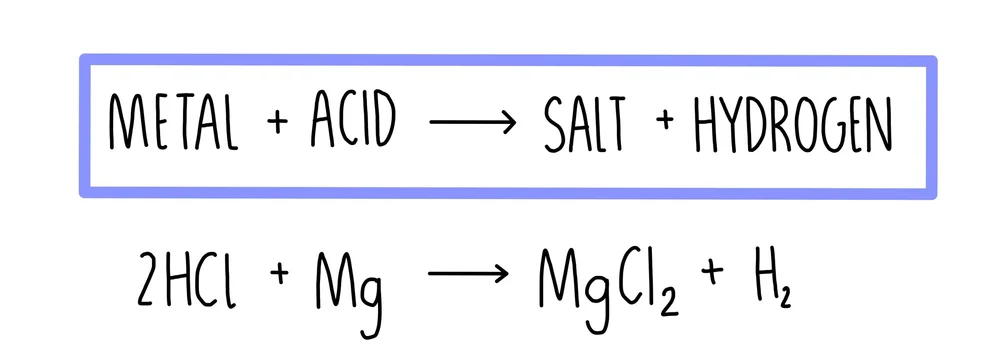
This diagram summarises the general reaction of a metal with a dilute acid to form a salt and hydrogen gas. The example illustrates magnesium reacting with hydrochloric acid to produce magnesium chloride and hydrogen. This directly reflects the metal–acid equation patterns required in OCR A-Level Chemistry. Source
To support these ideas, the following definition clarifies oxidation.
Oxidation: Loss of electrons by a species during a chemical reaction.
In metal–acid reactions, metals lose electrons to form positive ions, while hydrogen ions gain electrons to form hydrogen gas.
Why Hydrogen Gas Is Produced
Acids contain hydrogen ions (H⁺), which act as oxidising agents. Because metals donate electrons readily, these ions are reduced to hydrogen gas. The salt formed depends entirely on the anion present in the acid.
Identifying the Salt Formed
When naming the salt:
The first part of the salt’s name comes from the metal.
The second part comes from the acid’s anion.
Common patterns include:
Hydrochloric acid → chloride
Sulfuric acid → sulfate
Nitric acid → nitrate
These naming rules ensure consistency when constructing equation products.
Full Equations for Metal–Acid Reactions
The OCR specification emphasises full equations only, meaning that all reactants and products must appear as complete species.
Common Acids Used
Metals generally react with:
Hydrochloric acid (HCl)
Sulfuric acid (H₂SO₄)
Nitric acid (HNO₃)
Students must account for acid concentration and formulae when determining stoichiometric relationships.
General Equation Format
The general full equation appears below.
Metal–Acid Reaction (General) = Metal + Acid → Salt + Hydrogen
Metal = Solid element that donates electrons
Acid = Source of hydrogen ions in solution
Salt = Ionic product formed from metal cations and acid anions
Hydrogen = H₂ gas released during reaction
These equations demonstrate how oxidation and reduction occur simultaneously, even though ionic equations are not required.
A short descriptive explanation helps distinguish situations where deviations may occur. For instance, some metals such as copper do not react with dilute acids because they are less reactive than hydrogen and cannot displace it from solution.
Stoichiometric Considerations
Although the specification does not require ionic equations here, it is essential to write balanced equations. This involves balancing:
The atoms of each element
The charge where applicable (if metal forms multiple oxidation states)
The diatomic nature of hydrogen gas (H₂)
Balanced equations communicate the correct mole ratios needed for reaction understanding.
Redox Aspects of Metal–Acid Reactions
These reactions clearly illustrate redox behaviour:
Metal atoms are oxidised to metal cations.
Hydrogen ions are reduced to hydrogen gas.
Oxidation numbers are a useful tool for identifying these changes, though not explicitly required in equation writing for this subsubtopic.
Because these reactions always involve a transfer of electrons, they sit at the intersection of redox chemistry and acid reactions within the specification.
Factors Affecting Metal–Acid Reactivity
Several factors influence whether a reaction occurs and how vigorously it proceeds.
Position in the Reactivity Series
Metals higher in the series react more readily. For OCR A-Level, the key idea is that only metals more reactive than hydrogen displace hydrogen gas from acids.
Nature of the Acid
Different acids produce different salts:
HCl → chlorides
H₂SO₄ → sulfates
HNO₃ → nitrates
Dilute nitric acid behaves similarly to the others at this level, though concentrated nitric acid can exhibit oxidising behaviour beyond the scope of this subsubtopic.
Metal Charge and Ion Formation
Some metals form multiple oxidation states. It is important to write the correct salt formula that reflects the oxidation state adopted in the reaction.
Salt: An ionic compound formed when the hydrogen ion of an acid is replaced by a metal ion or ammonium ion.
Salts produced in metal–acid reactions are typically soluble and remain in solution after hydrogen gas escapes.
A metal forming a +2 ion will produce salts such as magnesium sulfate (MgSO₄), while a metal forming a +1 ion will form salts such as sodium chloride (NaCl). Students should confirm oxidation states when constructing final equations.
Observations During the Reaction
Metal–acid reactions typically display easily observable features:
Effervescence from hydrogen gas production
Temperature increase due to exothermic behaviour
Metal dissolution as the solid metal gradually disappears
Formation of an aqueous salt solution

This photograph shows magnesium ribbon reacting with dilute hydrochloric acid in a test tube. The bubbles represent hydrogen gas released as magnesium forms magnesium chloride. The page also mentions collecting and igniting hydrogen, which exceeds syllabus requirements, but the image itself remains directly relevant. Source
These observations provide useful qualitative evidence for the reaction and align with practical chemistry encountered in early laboratory experience.
Writing Accurate Full Equations
To meet OCR expectations, students must ensure:
Correct formulas for all reactants and products
Balanced equations with respect to atoms and hydrogen gas
Accurate salt naming corresponding to the acid used
Inclusion of hydrogen gas as H₂, not simply “H”
These principles ensure precision when writing full equations for reactions between metals and acids.
FAQ
Reaction rate depends on how readily a metal loses electrons. Metals high in the reactivity series have low ionisation energies and oxidise more easily, increasing hydrogen production.
Surface area, temperature and acid concentration also influence the rate. Powdered metals or freshly cleaned surfaces react faster because more particles are available for collision.
For the dilute acids used at A-Level, metals above hydrogen always form a salt and hydrogen gas.
Exceptions occur with strong oxidising acids such as hot, concentrated nitric acid, where nitrogen oxides may form instead of hydrogen. These reactions exceed OCR requirements but explain why nitric acid behaves differently under certain conditions.
Aluminium is protected by a thin, tough oxide layer that prevents contact between the metal and the acid.
Once this oxide layer is removed or disrupted, aluminium reacts vigorously and follows the standard pattern of producing a salt and hydrogen gas.
Observable differences include:
The speed and intensity of effervescence
The rate at which the metal dissolves
Temperature rise in the reaction mixture
A rapid reaction produces continuous, vigorous bubbling, while a slower reaction shows intermittent or gentle effervescence.
Hydrogen ions gain electrons to form hydrogen atoms, but these atoms are highly unstable and immediately pair up to form H2 molecules.
The formation of H2 is energetically favourable because the H–H bond provides stabilising energy, making diatomic hydrogen the universal product in metal–acid reactions.
Practice Questions
A student adds a piece of magnesium ribbon to excess dilute hydrochloric acid.
(a) State the products formed in this reaction. (1 mark)
(b) Write the balanced symbol equation for the reaction. (1 mark)
(2 marks)
(a)
Magnesium chloride and hydrogen gas. (1 mark)
(Allow: hydrogen; magnesium chloride.)
(b)
Balanced symbol equation: Mg + 2HCl → MgCl2 + H2 (1 mark)
(Equation must be correctly balanced and with correct formulae.)
Zinc reacts with dilute sulfuric acid in a typical redox reaction.
(a) Describe the observations you would expect to see when zinc reacts with dilute sulfuric acid. (2 marks)
(b) Explain, in terms of electron transfer, what happens to the zinc atoms and the hydrogen ions during this reaction. (2 marks)
(c) Write the balanced full equation for the reaction. (1 mark)
(5 marks)
(a)
Award up to 2 marks for:
Effervescence / bubbles of gas observed. (1 mark)
Zinc gradually dissolves / zinc solid gets smaller. (1 mark)
(Allow: mixture warms up — maximum 2 marks overall.)
(b)
Award up to 2 marks for:
Zinc atoms lose electrons and are oxidised to Zn2+. (1 mark)
Hydrogen ions gain electrons and are reduced to hydrogen gas. (1 mark)
(c)
Balanced equation: Zn + H2SO4 → ZnSO4 + H2 (1 mark)
(Equation must be correct and balanced.)

