OCR Specification focus:
‘Group 2 elements react with water and dilute acids, producing hydroxides or salts and hydrogen; relative rates illustrate increasing reactivity down the group.’
Reactions of Group 2 Metals with Water and Dilute Acids
Group 2 metals show clear, progressive trends in their reactions with water and dilute acids, offering strong evidence for increasing reactivity down the group. These reactions form characteristic hydroxides, salts, and hydrogen gas, and their relative rates provide essential insight into periodic behaviour. Understanding these reactions helps explain why Group 2 metals are widely used in industrial processes, laboratory settings, and qualitative analysis.
Reactivity of Group 2 Metals with Water
General Behaviour
Group 2 elements (Mg, Ca, Sr, Ba) react with water to produce metal hydroxides and hydrogen gas. These reactions become progressively more vigorous as atomic number increases, reflecting the trend of decreasing ionisation energy down the group.
Down the group, metals lose their outer s² electrons more easily.
Resulting 2+ ions form alkaline hydroxides, which vary in solubility and alkalinity.
Observable differences in reaction rate form an important comparative tool.
Reaction Pattern
All Group 2 metals follow the same fundamental pattern when reacting with liquid water:
Metal + water → metal hydroxide + hydrogen gas
Hydroxide formed is typically a white solid or sparingly soluble alkaline solution.
The increasing rate of reaction provides clear evidence for periodic trends.
Cold Water vs Steam
Not all Group 2 metals react with cold water:
Magnesium reacts very slowly with cold water, producing minimal hydrogen, but reacts readily with steam.
Calcium, strontium, and barium react increasingly vigorously with cold water.
A visible progression in effervescence and heat generation demonstrates rising reactivity.
Magnesium and Steam
Magnesium’s reactivity with steam is frequently tested due to the clear contrast between reactions with steam and cold water.
Steam: Water in its gaseous state, used to provide sufficient activation energy for reactions requiring higher temperatures.
When magnesium reacts with steam, it forms magnesium oxide instead of magnesium hydroxide.
A sentence is required here before introducing any equation.
Magnesium with Steam (Mg + H₂O(g)) = MgO + H₂
MgO = Magnesium oxide, a white solid
H₂ = Hydrogen gas
Observations and Trends
Key visual indicators when Group 2 metals react with water include:
Increasing effervescence due to hydrogen release
Faster dissolution or breakdown of the metal surface
Formation of white suspensions of hydroxides
Increasing alkalinity of the resulting solution down the group
Barium shows the most rapid and exothermic reaction, illustrating the strong increase in reactivity.
Overall, Group 2 metals react with cold water to form metal hydroxides and hydrogen gas, with reactivity increasing down the group from magnesium to barium.
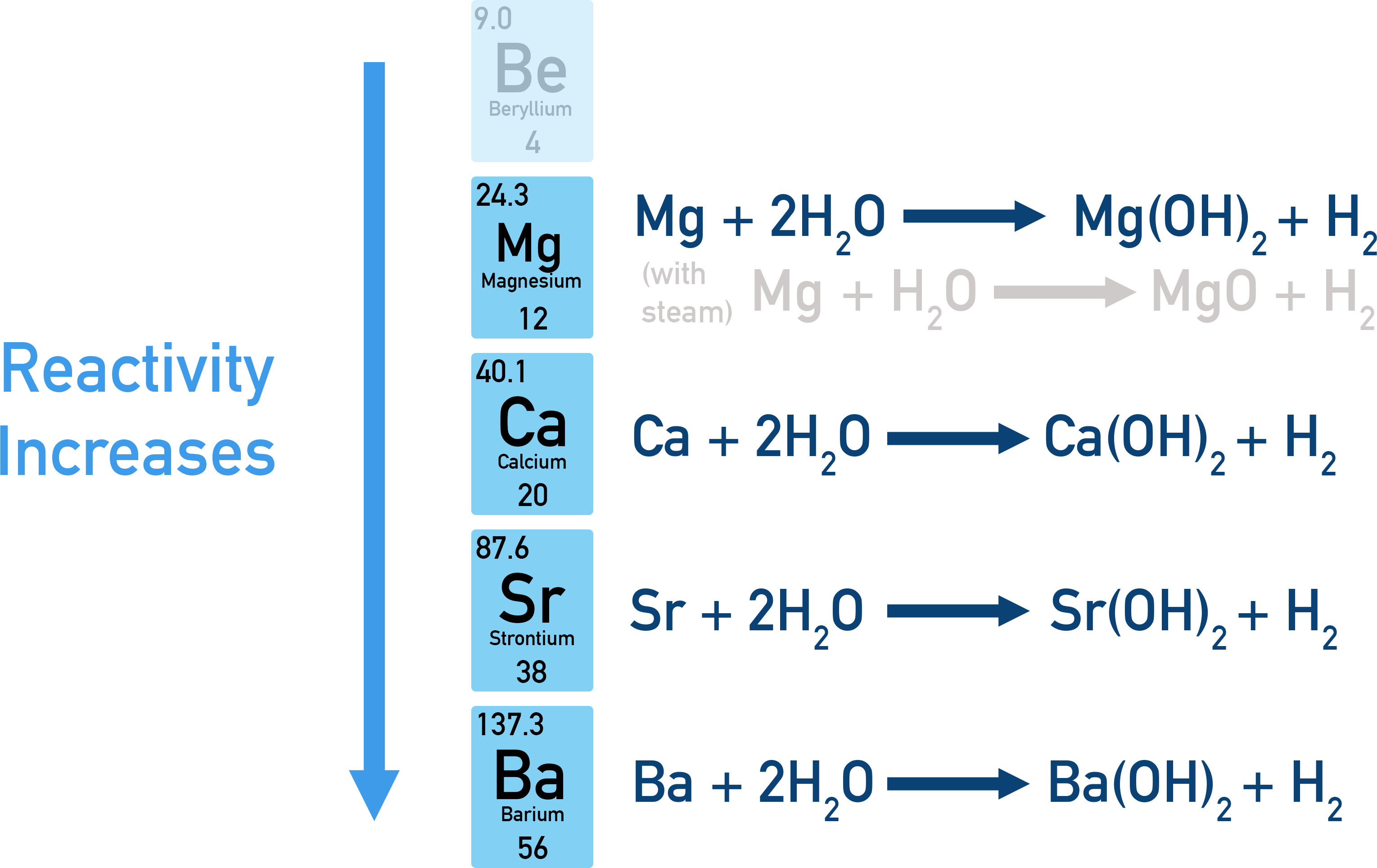
Diagram illustrating the reactions of Group 2 metals with water and the increase in reactivity down the group, showing the formation of metal hydroxides and hydrogen gas. Source
Reactions of Group 2 Metals with Dilute Acids
General Behaviour with Acids
All Group 2 metals react with dilute acids to form salts and hydrogen gas. These reactions are typically more vigorous than reactions with water because acids provide H⁺ ions that are readily reduced.
Reaction rates increase significantly down the group.
Products depend on the acid used, but all reactions produce a Group 2 salt.
Reaction Pattern
Group 2 metals follow a consistent pattern when reacting with dilute acids:
Metal + dilute acid → metal salt + hydrogen gas
This reaction is a classic example of a redox process, with the metal undergoing oxidation and H⁺ ions undergoing reduction.
Oxidation and Reduction
When a Group 2 metal reacts with an acid, its atoms lose electrons to form M²⁺ ions, while hydrogen ions gain electrons to become hydrogen gas.
Oxidation: Loss of electrons by a species during a chemical reaction.
A normal sentence must separate definition blocks, so here is one describing reduction.
Reduction: Gain of electrons by a species during a chemical reaction.
Examples of Acid Reactions
Typical dilute acids used include hydrochloric acid and sulfuric acid, producing corresponding Group 2 salts:
With hydrochloric acid → metal chlorides
With sulfuric acid → metal sulfates
As the reactions become more vigorous down the group, the rate of hydrogen production increases accordingly.
Solubility Considerations
The reaction of Group 2 metals with dilute sulfuric acid displays a complication due to solubility trends:
Magnesium sulfate and calcium sulfate are soluble enough to allow the reaction to continue.
Barium sulfate is highly insoluble and forms a surface layer on the metal.
This insoluble coating slows or eventually stops the reaction by preventing further acid contact.
This exception demonstrates the importance of solubility when predicting reaction behaviour.
Comparing Water and Acid Reactions
Key differences between the reactions include:
Dilute acids react far more readily than water because they supply H⁺ ions, which are stronger oxidising agents than water molecules.
Even relatively unreactive metals like magnesium react rapidly with dilute acids.
Down the group, increased reactivity results in noticeably faster effervescence and quicker formation of salts.
When Group 2 metals react with dilute acids such as hydrochloric or sulfuric acid, they form aqueous metal salts and hydrogen gas, often with vigorous effervescence.

Photograph showing magnesium reacting with dilute hydrochloric acid to release hydrogen gas, illustrating the general reaction pattern of Group 2 metals forming salts and hydrogen. Source
Key Trends Across Both Reaction Types
To interpret the behaviour of Group 2 metals accurately, focus on the following observable patterns:
Increasing reactivity from Mg → Ba
More vigorous hydrogen release down the group
More soluble hydroxides formed as atomic radius increases
Formation of corresponding salts in acid reactions
Impact of solubility of the products on reaction completion
These reactions provide fundamental evidence for the periodic behaviour of Group 2 metals and directly support the OCR specification requirements.
FAQ
The solubility of Group 2 hydroxides increases from magnesium to barium. As solubility increases, more hydroxide ions dissolve into the solution, raising the pH and producing a more strongly alkaline mixture.
This greater availability of hydroxide ions also explains why later Group 2 hydroxides are commonly used in processes requiring stronger alkaline conditions.
Increasing surface area increases the rate because more metal atoms are exposed to react with water molecules or hydrogen ions.
Finely divided metals or filings react significantly faster than larger pieces due to a greater number of collision sites and improved contact with the reactant.
Strong acids fully dissociate, providing a high concentration of hydrogen ions, so reactions are much faster.
Weak acids produce fewer hydrogen ions, resulting in slower rates.
Reactivity trends down Group 2 remain the same, but the observed intensity of effervescence is lower with weaker acids.
Reactions may release hydrogen gas rapidly, which is flammable.
Wear eye protection, use small quantities of metals, and keep ignition sources away.
For faster-reacting metals such as calcium, strontium, or barium, the reaction should be carried out behind a safety screen and using dilute acids to control the rate.
Cold water provides insufficient energy for the reaction to proceed appreciably, so only a limited amount of magnesium hydroxide forms at the surface and the reaction is slow.
Steam provides enough thermal energy to drive the reaction fully, forming magnesium oxide rather than the hydroxide because the high temperature favours the oxide product energetically.
Practice Questions
Magnesium reacts only very slowly with cold water but reacts readily with steam.
Explain why magnesium reacts much more rapidly with steam than with cold water.
(2 marks)
Steam provides more energy / higher temperature, allowing the magnesium to react more readily (1)
Magnesium forms magnesium oxide with steam because the activation energy barrier is more easily overcome (1)
Group 2 metals show clear trends in their reactions with water and dilute acids.
Using your knowledge of atomic structure and ionisation energies, explain:
a) Why calcium reacts more vigorously with cold water than magnesium.
b) Why barium reacts more vigorously with dilute hydrochloric acid than calcium.
c) Why the reaction between barium and dilute sulfuric acid slows down significantly as it proceeds.
(5 marks)
a) Why calcium reacts more vigorously with cold water than magnesium:
Calcium has a larger atomic radius and more shielding than magnesium (1)
Outer electrons are lost more easily / lower first ionisation energy (1)
b) Why barium reacts more vigorously with dilute hydrochloric acid than calcium:
Barium has an even larger atomic radius and increased shielding compared with calcium (1)
Even lower ionisation energy, so electrons are lost more readily, increasing reaction rate (1)
c) Why the reaction between barium and dilute sulfuric acid slows significantly:
Insoluble barium sulfate forms a coating on the metal surface (1)
This coating prevents further acid from contacting the metal, reducing the rate of reaction (1)

