OCR Specification focus:
‘Increasing atomic radius and shielding lower first and second ionisation energies down Group 2, explaining increased reactivity.’
Reactivity in Group 2 increases down the group because ionisation energies decrease. Understanding how atomic radius and electron shielding influence these energies explains the observed trend in chemical behaviour.
Explaining Reactivity Trends Using Ionisation Energies
Why Ionisation Energy Governs Reactivity in Group 2
Group 2 metals undergo oxidation in reactions, losing two electrons to form 2+ ions. The ease with which these electrons are lost is determined by their first and second ionisation energies. Lower ionisation energies make electron loss easier, increasing the metal’s reactivity.
Ionisation Energy: The energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of gaseous ions.
A decrease in ionisation energy down the group makes each successive element more reactive in processes such as reactions with water, oxygen, and dilute acids.
Factors Affecting Ionisation Energies Down the Group
1. Increasing Atomic Radius
As you move from magnesium to barium, atoms contain more electron shells. This increases the atomic radius, meaning the outer electrons are further from the nucleus.
A greater distance reduces the electrostatic attraction between the nucleus and the outer electrons.
Weaker attraction means electrons are removed more easily.
This contributes directly to the observed decrease in both first and second ionisation energies.
The influence of atomic radius becomes progressively more significant with each additional shell.
2. Increased Electron Shielding
Moving down Group 2 adds full inner electron shells, increasing electron shielding. Electrons in inner shells repel outer-shell electrons, reducing the effective nuclear charge experienced by those outer electrons.
Electron Shielding: The repulsion between electrons in different shells, which reduces the net attractive force from the nucleus on outer electrons.
Down Group 2, the number of filled inner shells increases, so electron shielding also increases.
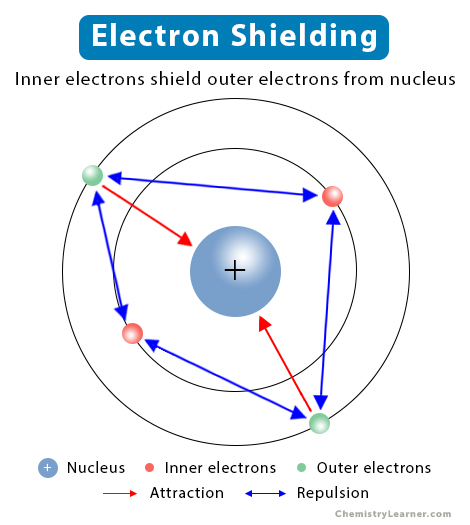
Schematic representation of electron shielding showing inner electrons reducing nuclear attraction on outer electrons; includes general atomic detail beyond OCR requirements. Source
As shielding increases, the nucleus cannot hold the outer electrons as tightly, further lowering the ionisation energies required to remove them.
A key point is that, although nuclear charge also increases down the group, the combined effects of larger atomic radius and stronger shielding outweigh this, leading to the overall decrease in ionisation energies.
First and Second Ionisation Energies in Group 2
First Ionisation Energy
This refers to removing the first s-electron from the outer shell. Down the group:
Atomic radius increases.
Shielding increases.
Attraction between the nucleus and outer electrons decreases.
First ionisation energy decreases steadily.
Experimentally, the first ionisation energy values for Be, Mg, Ca, Sr and Ba show a clear decrease down the group
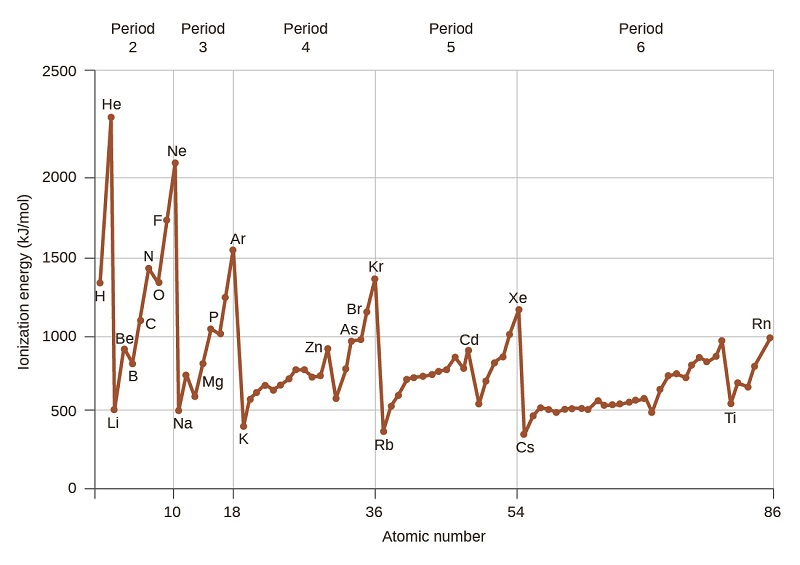
Graph showing periodic variation in first ionisation energy; includes additional group trends not required for OCR but clearly marks Group 2 elements to illustrate decreasing ionisation energy down the group. Source
A lower first ionisation energy means the initial electron is more easily removed, accelerating the oxidation process that begins many Group 2 reactions.
Second Ionisation Energy
The second ionisation energy involves removing the second outer-shell electron. This is still taken from the same s-sub-shell as the first, so:
The same shielding and radius trends apply.
Second ionisation energy also decreases down the group.
Even though the second electron is removed from a positively charged ion (M⁺), which increases the energy needed compared with the first electron, the trend still shows a clear decrease from Mg to Ba.
A consistently lower second ionisation energy is crucial because forming a 2+ ion requires both outer electrons to be removed.
Linking Ionisation Energies to Reactivity Trends
Why Reactivity Increases Down the Group
Group 2 reactivity is based on how readily atoms lose their two s-electrons. As both ionisation energies decrease down the group:
Less energy input is needed for oxidation.
Metals react faster and more vigorously with reagents such as water and acids.
The trend becomes more pronounced from Mg to Ba.
The metals’ ability to form 2+ ions with increasing ease is central to understanding their chemical behaviour.
Reactivity and Specific Group 2 Reactions
The decrease in ionisation energies explains characteristic reactivity patterns:
Reaction with water:
Magnesium reacts slowly (or not at all with cold water).
Calcium reacts noticeably faster.
Strontium and barium react vigorously.
Reaction with dilute acids:
All produce hydrogen gas and a salt, but the rate increases significantly down the group.
These patterns correlate with how easily electrons are lost during oxidation.
Interplay Between Nuclear Charge, Radius, and Shielding
Although nuclear charge increases down Group 2, it does not result in higher ionisation energies. The increase in nuclear charge is outweighed by:
A larger atomic radius, reducing electrostatic attraction.
Increased shielding from additional inner shells.
Effective Nuclear Charge: The net positive charge experienced by outer electrons after accounting for shielding by inner electrons.
Because effective nuclear charge does not increase enough to compensate for distance and shielding effects, ionisation energies fall, leading to higher reactivity.
Between any two definition or equation blocks, a normal sentence must appear, and here the explanation reinforces why ionisation energy trends dominate Group 2 behaviour.
Summary of Key Points for OCR-Level Understanding
Reactivity in Group 2 increases down the group because ionisation energies decrease.
Larger atomic radius and greater electron shielding are the primary causes of this decrease.
Lower first and second ionisation energies mean electron loss is easier, promoting formation of 2+ ions.
Observed increases in reaction rates with water, oxygen, and acids reflect these ionisation trends.
FAQ
Effective nuclear charge is the net attraction experienced by outer electrons after shielding is accounted for.
In Group 2, although nuclear charge increases down the group, shielding increases faster. This means the effective nuclear charge felt by the outer electrons does not rise enough to counter the increased atomic radius.
As a result, the outer electrons experience weaker attraction and are more easily removed.
Once the first electron is removed, the atom becomes a positively charged ion. Removing a second electron requires overcoming the attraction between the nucleus and this now more positively charged species.
However, despite the higher energy needed, the second ionisation energy still decreases down the group because:
Atomic radius increases
Shielding increases
Nuclear attraction to the outer electrons weakens
This allows Group 2 metals to form 2+ ions more easily down the group.
All Group 2 elements lose electrons from the same type of sub-shell: the outer s-subshell.
Because the first two electrons are always removed from an s-orbital:
Sub-shell differences have minimal influence compared with Groups 13–15
Trends are mainly governed by radius and shielding
The lack of sub-shell anomalies makes Group 2 ionisation trends smoother than across a period
This consistency helps produce a clear increase in reactivity down the group.
Magnesium has an electron configuration of 1s2 2s2 2p6 3s2.
Its first two ionisations remove the 3s electrons. The third ionisation would remove an electron from the fully filled 2p subshell, which is closer to the nucleus and experiences much stronger attraction.
This large jump in required energy shows that magnesium naturally forms only a 2+ ion.
Ionisation energies can be used to anticipate:
Reaction rate
Vigour of hydrogen production
Temperature needed to initiate reaction
For example, a metal with lower ionisation energies will:
React faster with water
Require less heating to react with oxygen
Produce more rapid effervescence with acids
This allows chemists to choose appropriate conditions when handling different Group 2 elements.
Practice Questions
Explain why the first ionisation energy decreases down Group 2.
(2 marks)
Atomic radius increases down the group, so outer electrons are further from the nucleus (1 mark).
Increased electron shielding reduces the nuclear attraction to the outer electrons, making them easier to remove (1 mark).
Magnesium reacts less vigorously with water than barium.
Using ideas about atomic structure and ionisation energies, explain why the reactivity of Group 2 metals increases from magnesium to barium.
Your answer should refer to both the first and second ionisation energies.
(5 marks)
Award marks for the following points (max 5):
Down the group, atoms have more electron shells and a larger atomic radius (1 mark).
Increased electron shielding from additional inner shells reduces the effective nuclear charge experienced by outer electrons (1 mark).
The attraction between the nucleus and the outer electrons decreases (1 mark).
This causes both the first and second ionisation energies to decrease from magnesium to barium (1 mark).
Lower ionisation energies mean the two outer electrons are more easily lost, so oxidation occurs more readily, increasing reactivity down the group (1 mark).

