OCR Specification focus:
'Group 2 metals have outer-shell s2 electrons and typically lose both in redox reactions to form 2+ ions.'
Introduction
Group 2 elements share a characteristic s² valence-electron configuration, leading to similar reactivity patterns and predictable formation of 2+ cations through electron loss in redox processes.
The s² Electron Configuration in Group 2
Group 2 elements, also known as the alkaline earth metals, are defined by their outer-shell electron configuration of ns². This configuration underpins their behaviour in chemical reactions and explains the consistent formation of 2+ ions across the group.
Electron configuration: The arrangement of electrons in atomic orbitals, showing how electrons occupy shells and sub-shells.
The ns² configuration means that each Group 2 atom possesses two electrons in its highest-energy s-sub-shell. These electrons experience relatively low effective nuclear attraction compared with inner-shell electrons, making them more accessible for removal during chemical reactions. This accessibility of valence electrons is a key reason why Group 2 elements largely act as reducing agents, giving electrons away in reactions.
Alkali metals (Group 1) and alkaline earth metals (Group 2) highlighted on the periodic table, illustrating their shared group characteristics and consistent s² configurations. Source
Trends Across the Group
Atomic Structure Factors
Down Group 2, atomic radius increases as additional electron shells are occupied. This expansion weakens the attraction between the nucleus and the valence electrons. Shielding by inner shells also increases, further reducing nuclear pull on the s² electrons. As a result, the ease with which these electrons are removed increases progressively down the group.
Reactivity Implications
These structural features lead to a clear reactivity trend:
Metals lower in the group react more vigorously.
Electron removal becomes easier, facilitating faster redox reactions.
The formation of 2+ ions becomes increasingly energetically favourable.
Formation of 2+ Ions
The Group 2 metals typically undergo oxidation by losing both of their outer-shell electrons:
Loss of one electron forms a +1 state.
Loss of the second electron produces the characteristic 2+ ion, resulting in a stable noble-gas configuration.
Oxidation: The loss of electrons by a species in a chemical reaction, increasing its oxidation number.
This electron loss is central to redox chemistry and underpins the metals’ characteristic behaviours.
A brief sentence here ensures clarity before including an equation block.
Formation of a 2+ ion (M) = M → M²⁺ + 2e⁻
M = Group 2 metal atom
e⁻ = Electron
Why 2+ Ions Form Consistently
The consistency of 2+ ion formation arises from:
The energetic favourability of removing the paired s-electrons.
The substantial stability gained from achieving a noble-gas configuration.
The lack of need to remove electrons from inner shells, which would require significantly more energy.
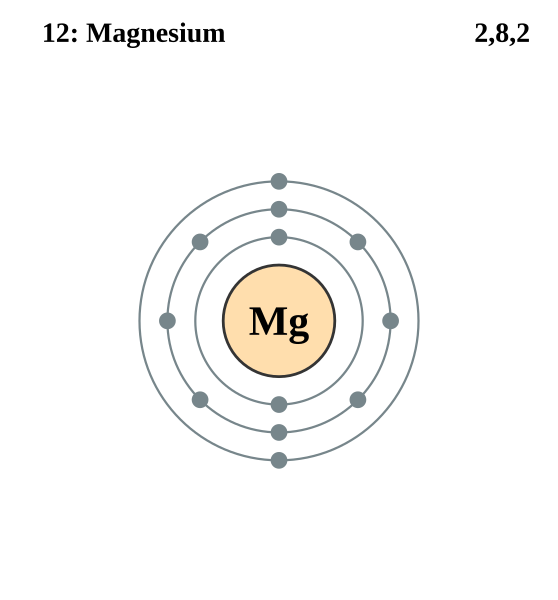
Electron shell diagram for magnesium showing 2, 8 and 2 electrons, illustrating the accessible s² valence electrons characteristic of Group 2 elements. Source
Redox Behaviour
Role as Reducing Agents
Group 2 metals act as strong reducing agents, because they readily donate electrons. Their tendency to lose electrons in reactions causes the species they react with to be reduced.
Magnesium and calcium commonly display this behaviour in reactions with oxygen, water, and acids.
Down the group, reducing strength increases as electron removal becomes easier.
Oxidation States
The metals adopt an oxidation state of +2 in virtually all their common compounds. Because the ns² electrons are the only accessible valence electrons, higher oxidation states are not generally observed.
Electron Removal and Ionisation
First and Second Ionisation
Although full ionisation-energy trends are covered elsewhere, it is essential here to understand that:
The first ionisation energy involves removing the first s-electron.
The second ionisation energy removes the remaining s-electron.
Both energies decrease down the group due to increased shielding and atomic radius.
This facilitates the formation of M²⁺ ions during reactions.
Ionisation energy: The energy required to remove one mole of electrons from one mole of gaseous atoms or ions.
Ionisation principles reinforce why Group 2 metals favour the +2 ion rather than forming multiple oxidation states like transition metals.
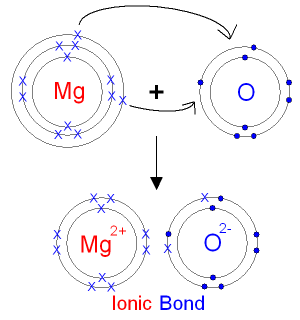
Diagram showing magnesium losing two valence electrons to form Mg²⁺, emphasising the oxidation process and consistent 2+ ion formation in Group 2 metals. Source
Properties Resulting from s² Loss
Ionic Structure
Group 2 ions form ionic compounds with non-metals, and these compounds characteristically contain M²⁺ cations paired with anions such as O²⁻, Cl⁻ or SO₄²⁻.
Lattice Formation
The 2+ charge contributes to strong electrostatic attraction in ionic lattices, generating:
High melting points
High boiling points
Typical solubility patterns across the group
Stability of the M²⁺ Ion
The stable noble-gas electron configuration reached after losing two electrons explains:
The predominance of M²⁺ ions in natural and manufactured compounds
The strongly exothermic formation of ionic lattices
The minimal tendency of Group 2 metals to form alternative oxidation states
Key Features of Group 2 s² Configuration
All Group 2 elements have an ns² outer-shell arrangement.
They characteristically lose both electrons to form 2+ ions.
Reactivity and ease of ion formation increase down the group.
Resulting ions have stable noble-gas configurations.
Redox behaviour is dominated by oxidation and electron donation.
FAQ
The s2 configuration means each atom has two electrons in a higher-energy sub-shell that are relatively easy to remove.
As you go down the group:
Increased shielding reduces the attraction between the nucleus and the s-electrons.
A larger atomic radius means outer electrons are further away.
These factors combine to lower both first and second ionisation energies, making electron loss more favourable.
Removing more than two electrons would require breaking into a full inner shell, which demands very high energy.
Group 2 metals therefore stop at losing their two s-electrons because:
The energy cost beyond 2+ is extremely high.
The noble-gas configuration gained at 2+ is already very stable.
This makes +2 the overwhelmingly preferred oxidation state.
Metallic bonding strength is partly influenced by the number of delocalised electrons.
Group 2 atoms contribute two delocalised electrons per ion, rather than one as in Group 1, giving:
Higher charge density
Stronger attraction between ions and electron sea
Higher melting points and hardness compared with Group 1 metals
These bonding differences arise directly from the s2 configuration.
When a Group 2 metal loses its two outer-shell electrons, the resulting ion has the electron configuration of the nearest noble gas.
This creates:
A stable, full outer shell
Strong electrostatic attraction when forming ionic lattices
Minimal tendency for further electron loss or gain
The change to a noble-gas configuration is the key stabilising factor.
Group 2 metals readily donate their two s-electrons in redox reactions.
Their effectiveness comes from:
Low ionisation energies relative to many other metals
High tendency to form stable 2+ ions
Ease of transferring two electrons in a single reaction step
Because they undergo oxidation so easily, they strongly drive reductions in other species.
Practice Questions
Group 2 elements form 2+ ions in their reactions.
Explain why a Group 2 atom forms a 2+ ion, referring to its electron configuration.
(2 marks)
1 mark: States that Group 2 atoms have an outer electron configuration of s2 or two electrons in the outer shell.
1 mark: Explains that they lose both electrons to achieve a stable noble-gas configuration, forming a 2+ ion.
Magnesium reacts readily with dilute acids to form magnesium salts and hydrogen gas.
This behaviour is linked to its electron configuration and the ease with which it forms Mg²⁺ ions.
Discuss, in terms of electron configuration, ion formation, and atomic structure down Group 2, why:
(a) Magnesium forms Mg²⁺ ions in redox reactions, and
(b) Metals lower in Group 2 (such as calcium and strontium) are more reactive than magnesium.
(5 marks)
(a) Magnesium forming Mg²⁺ ions:
1 mark: Magnesium has two electrons in its outer shell (3s2).
1 mark: It loses both electrons in oxidation to form Mg²⁺.
1 mark: This results in a stable electron configuration (same as a noble gas).
(b) Increasing reactivity down the group:
1 mark: Atomic radius increases and shielding increases down the group.
1 mark: Weaker nuclear attraction makes removal of outer electrons easier, so Ca and Sr lose their s2 electrons more readily than magnesium.

