OCR Specification focus:
‘Halogens have outer s2p5 electron configurations and often gain one electron in redox reactions to form 1– halide ions.’
Introduction
Halogens display characteristic electron configurations and redox behaviours that underpin their reactivity. Understanding their s2p5 arrangement explains electron-gain tendencies and the formation of halide ions in redox processes.
Electron Configuration of the Halogens
Halogens (Group 17) share a distinctive outer-shell electron structure, which is fundamental to explaining their chemical behaviour.
The s2p5 Arrangement
All halogens possess an outer shell with seven electrons, arranged as s2p5. This configuration places them one electron short of a full p subshell, driving strong tendencies toward electron gain.
Electron Configuration: The arrangement of electrons in shells and subshells around the nucleus of an atom.
Because halogens need only one additional electron to complete their outer shell, they commonly undergo reactions that facilitate this gain. These processes directly link their electronic structure to their redox properties.
All the halogens have the outer-shell configuration ns² np⁵, giving seven valence electrons which strongly influence their chemical behaviour.
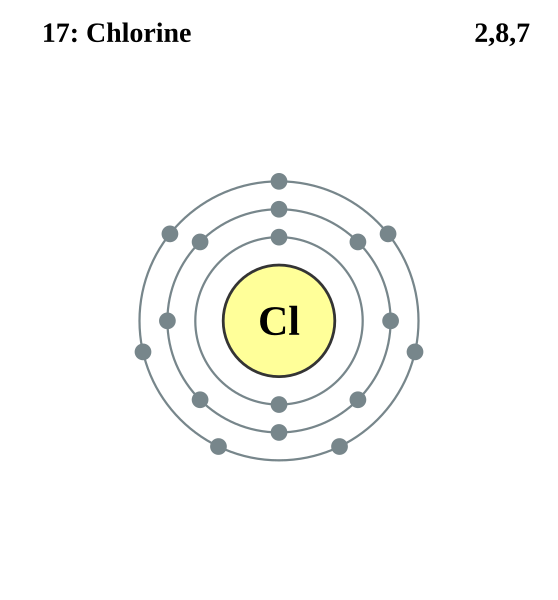
Electron shell diagram for chlorine, showing electrons arranged in shells. The outer shell contains seven electrons, illustrating the ns² np⁵ configuration. This reinforces the relationship between valence electrons and halogen redox behaviour. Source
A sentence here ensures spacing before the next definition block.
Halogens: A group of non-metallic elements in Group 17, characterised by an outer-shell s2p5 electron configuration and high reactivity.
Their shared electron structure results in similar patterns of reactivity while still allowing differences in oxidising ability down the group.
Subshell Structure and Periodic Trends
Across Period 3 and downward through the group, increased atomic radius, additional electron shells, and greater shielding influence halogen behaviour. The larger the halogen atom, the weaker its attraction for incoming electrons, affecting both redox strength and reactivity.
Redox Behaviour of the Halogens
The halogens typically act as oxidising agents, meaning they gain electrons from other species during reactions.
Oxidising Agents and Electron Gain
Halogens readily undergo reduction, gaining one electron to form a 1– halide ion. This behaviour reflects the specification requirement that halogens “often gain one electron in redox reactions to form 1– halide ions.”
Redox Reaction: A chemical process involving simultaneous reduction (electron gain) and oxidation (electron loss).
Halogens oxidise other species during these reactions by removing electrons from them.
A follow-up sentence maintains separation before the next definition block.
Reduction: The gain of electrons by a species during a chemical reaction.
This strong tendency for reduction is most pronounced in fluorine, the most electronegative halogen, and decreases down the group as atoms become larger and more shielded.
Formation of Halide Ions
Each halogen forms a corresponding halide ion by accepting a single electron:
Chlorine → Cl⁻
Bromine → Br⁻
Iodine → I⁻
This electron acceptance completes the outer shell, giving the halide ion a stable noble-gas configuration.
Halide ions are common in natural salts, displacement reactions, and qualitative analysis tests, and their formation is fundamental to halogen redox chemistry.
In a typical redox change, a halogen molecule X₂ is reduced to two halide ions, 2X⁻, while the other reactant is oxidised and loses electrons.
Diagram showing electron transfer in the reaction between sodium and chlorine. Sodium loses electrons to form Na⁺ while chlorine gains electrons to form Cl⁻. This illustrates how halogens act as oxidising agents by undergoing reduction to halide ions. Source
Electron Gain and Atomic Properties
Understanding why halogens gain electrons requires considering nuclear charge, distance, and shielding effects.
Nuclear Charge
Halogens possess relatively high nuclear charge, exerting strong attraction on additional electrons. As atomic number increases down the group, nuclear charge increases, but this is offset by shell expansion.
Atomic Radius and Shielding
Moving down the group:
More shells increase atomic radius
Greater inner electron shielding weakens the pull on electrons
Reduced attraction lowers oxidising ability
These factors explain why fluorine is a stronger oxidising agent than chlorine, and chlorine stronger than bromine or iodine.
Redox Patterns Down the Group
Trends in redox behaviour follow predictable patterns derived from the electron configuration and structure of each halogen.
Decreasing Oxidising Power
Oxidising strength diminishes down the group because larger atoms cannot attract electrons as effectively. This trend influences:
Displacement reactions
Reaction rates
Halogens’ ability to oxidise halide ions
These outcomes are directly tied to the s2p5 electron configuration and resulting electron affinity characteristics.
Relationship Between Electron Configuration and Redox Strength
Key factors linking configuration to redox activity include:
Energy required to add an electron
Amount of shielding from inner shells
Electronegativity differences across the group
Because all halogens need just one electron, their behaviours show continuity, but electron-gain ease varies substantially with atomic size.
Summary of Key Behavioural Features
Although no conclusion is required, the following core ideas integrate specification-required knowledge:
Essential Characteristics
Halogens possess outer-shell s2p5 configurations.
They typically gain one electron in redox reactions.
This gain forms 1– halide ions.
Redox trends stem from changes in radius, shielding, and nuclear attraction down the group.
These features make halogen chemistry predictable, enabling systematic understanding of their reactions and redox behaviour within Periodic Table trends.
FAQ
Halogens have a strong tendency to attract electrons because their outer-shell configuration is one electron short of a full p subshell. This creates a high effective nuclear attraction for incoming electrons.
Their ability to form 1– ions is enhanced by:
High electronegativity
Relatively high electron affinity
Low energy requirement for adding one electron compared with completing multiple vacancies, as seen in other p-block elements
Although nuclear charge increases from chlorine to iodine, additional electron shells significantly increase shielding. This shielding weakens the overall attraction between the nucleus and an incoming electron.
As atomic radius grows, the outer electrons are also further from the nucleus, reducing the energy released when an atom gains an electron.
Halogens exist as diatomic molecules, meaning redox changes typically involve the entire X2 unit rather than isolated atoms.
During reduction:
X2 gains two electrons in total
The molecule splits to form two X– ions
Bond energy must be overcome, affecting reaction conditions and overall feasibility
Fluorine’s exceptionally small atomic radius brings its valence electrons very close to the nucleus, increasing attraction for incoming electrons. It also experiences less repulsion when gaining an additional electron due to its compact subshell arrangement.
However, its high reactivity is further supported by:
High electronegativity
High electron affinity
Minimal shielding relative to other halogens
The p subshell in halogens is relatively low in energy, making it energetically favourable for an additional electron to enter and complete the subshell.
Energy factors influencing electron gain include:
Spacing of the p orbitals
Repulsion between existing p electrons
Relative stability gained by forming a full np6 configuration
These principles help explain why halogens are consistently good oxidising agents across the group.
Practice Questions
Chlorine has the outer-shell electron configuration 3s2 3p5.
(a) State the number of electrons chlorine needs to gain to achieve a full outer shell.
(b) State the species formed when chlorine gains this number of electrons.
(2 marks)
(a) 1 mark:
Chlorine needs to gain 1 electron.
(b) 1 mark:
The species formed is the chloride ion (Cl−).
The halogens show characteristic redox behaviour based on their electron configurations.
(a) Explain why halogens act as oxidising agents in redox reactions.
(b) Describe and explain the trend in oxidising strength as you go down Group 17 from chlorine to iodine.
(c) Write the half-equation for the reduction of bromine to bromide ions.
(5 marks)
(a) 2 marks:
Halogens act as oxidising agents because they gain electrons in reactions.
Their s2p5 electron configuration means they need only one electron to complete the outer shell.
(b) 2 marks:
Oxidising strength decreases down the group.
Increasing atomic radius and greater shielding reduce the attraction for an incoming electron.
(c) 1 mark:
Half-equation: Br2 + 2e− → 2Br−

