OCR Specification focus:
'Halogens exist as diatomic molecules. Boiling points increase from Cl₂ to I₂ due to stronger London dispersion forces.'
Introduction
Halogens show clear physical trends down the group, particularly in physical state, colour, and boiling point, all linked to molecular structure and intermolecular forces.
Halogen Molecular Structure and Physical Characteristics
Diatomic Molecular Form
All halogens exist naturally as diatomic molecules (X₂). Because they are simple, non-polar molecules, their physical properties arise from their molecular masses and the strength of intermolecular attractions.
Diatomic molecule: A molecule consisting of only two atoms chemically bonded, often of the same element.
These X–X covalent bonds are relatively strong, but the intermolecular attractions between molecules vary greatly down the group.
Trends in Physical State
Down Group 17, halogens show a distinct and predictable progression in physical form:
Chlorine (Cl₂): green–yellow gas at room temperature
Bromine (Br₂): dark red–brown volatile liquid
Iodine (I₂): grey–black crystalline solid producing purple vapours
The change from gas to liquid to solid reflects increasing intermolecular forces as atomic size increases.
Colour Changes
Halogens also deepen in colour down the group due to increasing numbers of electrons, which cause more complex electron transitions when absorbing visible light.
Lighter colours (Cl₂) indicate fewer available transitions.
Darker colours (I₂) reflect more extensive electron clouds.
Intermolecular Forces in Halogens
London Dispersion Forces
The primary intermolecular force between halogen molecules is London dispersion forces, a type of induced dipole–induced dipole attraction. These forces arise because electrons within molecules constantly move, creating temporary dipoles that induce dipoles in neighbouring molecules.
London dispersion forces: Weak intermolecular attractions caused by instantaneous and induced dipoles between molecules.
Although individually weak, these forces become stronger as molecular size and electron count increase.
A halogen molecule with more electrons fluctuates more significantly, producing larger temporary dipoles and stronger induced dipoles. This increased attraction means more energy is required to separate molecules during boiling.
Boiling-Point Trends Down Group 17
Increasing Boiling Points from Cl₂ to I₂
The OCR specification emphasises that boiling points increase from chlorine to iodine because London dispersion forces strengthen. This trend is essential for understanding Group 17 physical behaviour:
Cl₂: lowest boiling point (gas at room temperature)
Br₂: intermediate boiling point (liquid)
I₂: highest boiling point (solid)
As you descend the group:
Atomic radius increases.
Number of electrons increases.
Electron cloud becomes more polarisable.
Temporary dipoles become larger and more frequent.
London dispersion forces strengthen.
More energy is needed to overcome these forces.
Therefore, boiling point increases.
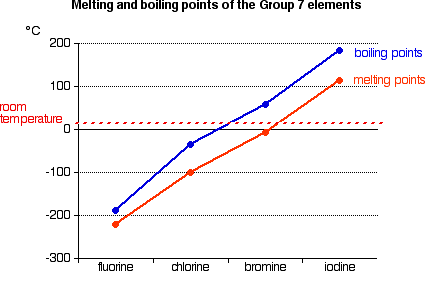
A graph displaying melting and boiling points of fluorine, chlorine, bromine, and iodine. It shows the increase in both melting and boiling points down the group. Melting point data are included as additional context beyond the OCR focus. Source
One normal sentence should occur here to maintain correct spacing before the next definition block.
Polarisability: The ease with which an electron cloud can be distorted to create temporary dipoles.
A more polarisable electron cloud leads to stronger induced dipoles, reinforcing the trend.
Relationship Between Molecular Mass and Boiling Point
Because halogens are diatomic, molecular mass directly influences boiling point. I₂ has the highest molecular mass, resulting in the greatest polarisability and strongest London dispersion forces.
Key factors contributing to increasing boiling point down the group:
Greater molecular mass → more electrons
Larger electron cloud → greater polarisability
Stronger intermolecular forces → higher boiling point
Observations in the Laboratory
Appearance of Halogens
Understanding physical observations assists in experimental recognition:
Cl₂ forms a pale green gas with a sharp, choking odour.
Br₂ appears as a brown liquid, giving off brown fumes.
I₂ is a metallic grey solid that sublimes to form purple vapour.

A photograph of chlorine, bromine, and iodine displayed in laboratory containers, highlighting their physical states and characteristic colours. From left to right: chlorine gas, bromine liquid, and iodine solid. Fluorine and astatine are absent because of handling hazards outside the OCR scope. Source
Volatility Decreases Down the Group
Volatility describes how readily a substance evaporates. Because stronger intermolecular forces require more energy to overcome, volatility decreases as boiling point increases:
Chlorine: very volatile (gas)
Bromine: moderately volatile (liquid with fumes)
Iodine: least volatile (solid)
Linking Structure, Intermolecular Forces, and Physical Trends
Structural Explanation
The halogens’ simple diatomic structure means their trends are governed mainly by intermolecular forces rather than internal bonding. Each molecule contains:
A single covalent bond between two atoms
A non-polar electron distribution
A tendency to form temporary dipoles
Since the covalent bond strength is relatively constant across the group, physical trends arise from differences in size and electron count, not from internal bonding changes.
Bullet-Point Summary of Key Connections
Halogens are diatomic, non-polar molecules.
London dispersion forces are the only significant intermolecular force.
Electron cloud size and molecular mass increase down the group.
Stronger dispersion forces require more energy to overcome.
Boiling points rise from Cl₂ → Br₂ → I₂.
Physical states change from gas → liquid → solid.
Colours darken as electron transitions become more complex.
These structural and energetic relationships underpin the OCR specification statement linking increasing boiling points to strengthening London dispersion forces.
Additional Observations Linked to Physical Trends
Solubility Considerations
Halogens’ solubility also changes with intermolecular forces. Although not a required focus, it supports understanding of physical behaviour:
Cl₂ dissolves moderately in water.
Br₂ dissolves less readily due to stronger intermolecular attractions.
I₂ has very limited solubility, forming only a slight brown solution unless iodide ions are present.
Melting Point Changes
While boiling points are the primary focus, melting points follow a similar trend for the same structural reasons. Stronger intermolecular forces mean more energy is needed for phase changes.
Final Connections to Specification Focus
The OCR specification highlights two essential features:
Halogens are diatomic molecules, explaining why their molecular features dominate their physical behaviour.
Boiling-point increases from Cl₂ to I₂ arise from progressively stronger London dispersion forces due to increased electron count and polarisability.
FAQ
Fluorine and astatine are less frequently included because fluorine is extremely reactive and difficult to handle, while astatine is highly radioactive and exists only in tiny, short-lived quantities.
As a result, practical data on their boiling points, colours and physical forms are limited, so OCR focuses on chlorine, bromine and iodine, where reliable observable trends exist.
A larger molecular surface area allows more contact between neighbouring molecules, strengthening instantaneous dipole interactions.
As halogens increase in size down the group, their surface area grows, enhancing London dispersion forces and raising boiling points.
As the number of electrons increases, halogen molecules absorb a broader range of visible wavelengths.
This leads to:
More complex electron transitions
Greater absorption of higher-energy light
Deeper observed colours
The trend reflects changes in electronic structure rather than differences in intermolecular forces.
Iodine’s lattice structure is held together by relatively weak intermolecular forces compared with typical ionic or metallic solids.
Because these forces are easier to overcome, iodine transitions directly from solid to gas when heated, producing its characteristic purple vapour.
Volatility decreases down the group because stronger London dispersion forces make it harder for molecules to escape into the gas phase.
Chlorine: very volatile due to weak intermolecular forces
Bromine: less volatile as forces strengthen
Iodine: low volatility as strong dispersion forces hold molecules in the solid state
Practice Questions
Chlorine has a much lower boiling point than iodine. Explain why chlorine boils at a lower temperature than iodine.
(2 marks)
Chlorine has fewer electrons / smaller electron cloud than iodine. (1)
Therefore chlorine has weaker London dispersion forces and requires less energy to separate its molecules. (1)
The halogens exist as diatomic molecules (X2) and show a clear trend in boiling points from chlorine to iodine.
(a) Describe how London dispersion forces arise between halogen molecules.
(b) Explain why the boiling points of the halogens increase from Cl2 to I2.
(c) Predict the physical state of bromine at room temperature and justify your answer using intermolecular forces.
(5 marks)
(a)
Temporary dipoles form when electrons move within a molecule. (1)
These induce dipoles in neighbouring molecules, creating London dispersion forces. (1)
(b)
Down the group, the number of electrons increases and electron clouds become more polarisable. (1)
Larger temporary dipoles form, so London dispersion forces become stronger. (1)
More energy is needed to separate the molecules, so boiling points increase. (1)
(c)
Bromine is a liquid at room temperature. (1)
Its intermolecular forces are stronger than those in chlorine but weaker than in iodine, giving a boiling point just above room temperature. (1)

