OCR Specification focus:
‘Decreasing ease of forming 1– ions down the group due to increased atomic radius and electron shielding reduces oxidising power.’
Halogen reactivity decreases down Group 17 because atomic size, shielding and electron-gain processes become less favourable, reducing each element’s ability to act as an effective oxidising agent.
Halogen Reactivity within Group 17
Halogen reactivity depends on how readily each element accepts an electron to form a 1– halide ion, and how strongly it can act as an oxidising agent. The OCR specification emphasises that the trend is controlled by changes in atomic radius, electron shielding, and the ease of forming halide ions. Understanding these factors allows students to explain why chlorine is more reactive than bromine, and why both are more reactive than iodine.
Fundamental Process: Forming Halide Ions
The key chemical process underpinning halogen reactivity is electron gain.
When a halogen atom gains one electron, it forms a halide ion with a full outer p-subshell. The ability of the atom to attract and accept this electron governs its oxidising strength.
Oxidising Agent: A species that gains electrons and causes another species to be oxidised.
The halogens act as oxidising agents during displacement reactions because they accept electrons from halide ions of less reactive elements.
Trend Explained by Atomic Radius
Moving down Group 17 from chlorine to bromine to iodine, the atomic radius increases. Additional electron shells mean the outer electrons are further from the nucleus, and the nuclear attraction experienced by incoming electrons is weaker. This decrease in attraction reduces the ability of each halogen to form a 1– ion, lowering its reactivity.
This weakening attraction directly links to the declining oxidising power emphasised in the specification. Down Group 17, atomic radius increases and there are more inner shells, so electron shielding also increases.
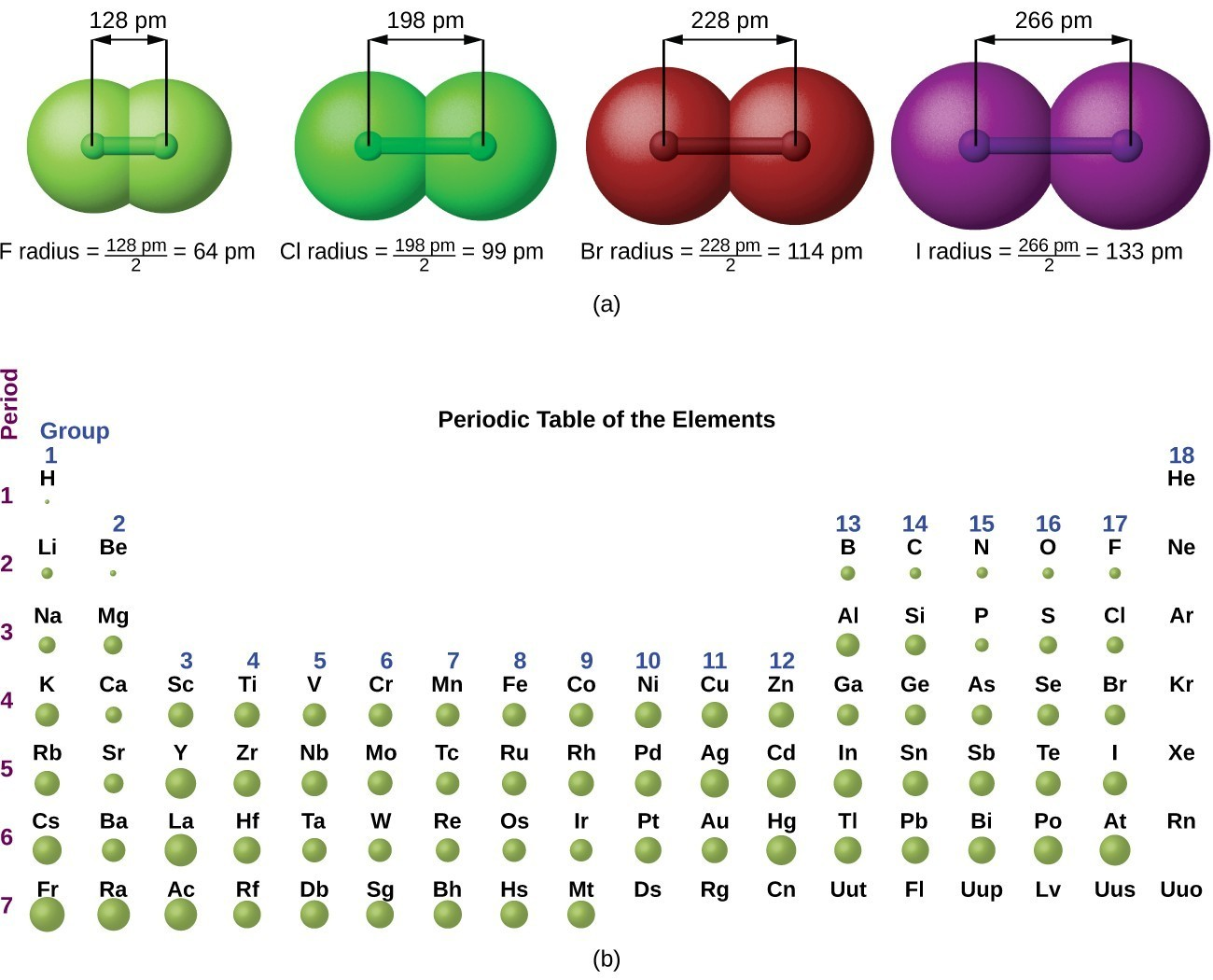
The diagram shows covalent radii and bond distances for the halogens, highlighting the increase in atomic radius down the group and supporting the trend in decreasing oxidising strength. Source
Electron Shielding and Its Effect
As more shells are added down the group, increased electron shielding reduces the effective nuclear charge felt by an incoming electron. The repulsion from inner shells counteracts the positive pull from the nucleus. The combined effect of increased radius and greater shielding means that gaining an electron becomes progressively more difficult.
These factors mean it becomes harder for larger halogen atoms to attract an incoming electron, so their oxidising power decreases down the group.
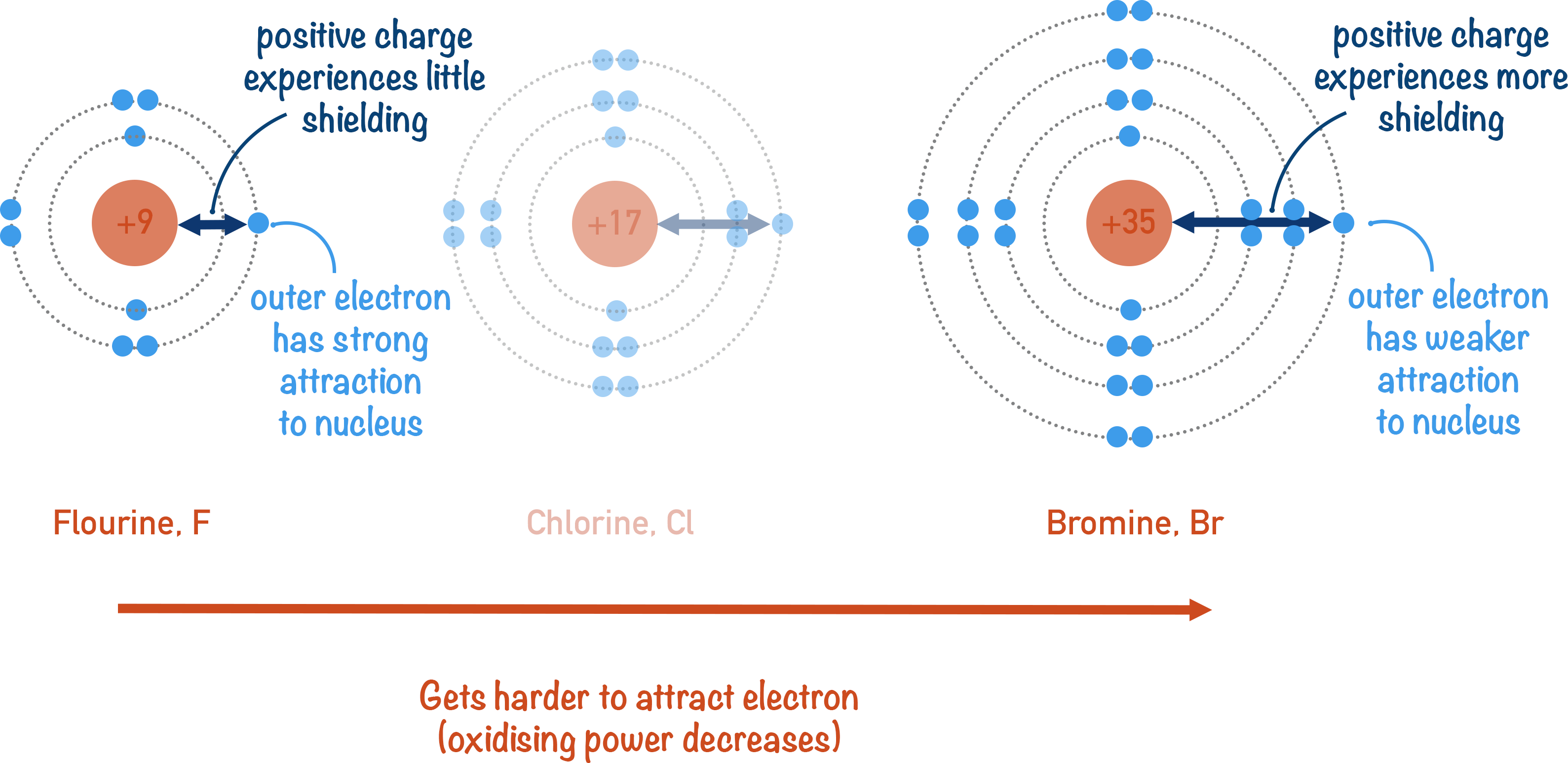
The image uses simplified electron-shell diagrams to illustrate how increasing shielding down the halogen group weakens nuclear attraction for incoming electrons, reducing oxidising ability. Source
Nuclear Charge: Why It Does Not Reverse the Trend
Although nuclear charge technically increases down the group, the large rise in shielding and atomic radius outweighs this factor. Therefore, the overall ability of halogens to attract additional electrons still declines down the group.
Energy Considerations in Electron Gain
Halogen reactivity is connected to how energetically favourable it is for an atom to accept an electron. The electron affinity—the enthalpy change when an atom gains an electron—generally becomes less exothermic down the group. Less energy is released when forming a halide ion, which reflects the reduced attraction for incoming electrons.
This matches the specification’s statement that the ease of forming 1– ions decreases from chlorine to iodine.
Oxidising Power and Displacement Reactions
Halogens participate in displacement reactions where a more reactive halogen replaces a less reactive halide ion in solution. The ease with which a halogen gains an electron determines its success in these reactions.
Because chlorine has the highest oxidising power of the three common halogens studied at A-level, it displaces both bromide and iodide ions. Bromine displaces only iodide ions, and iodine displaces none. These outcomes are consistent with the trend explained by atomic radius and shielding.
Link Between Structure and Oxidising Strength
The decreasing reactivity reflects fundamental atomic structure changes down the group.
Key structural features include:
Increasing number of electron shells
Greater shielding from inner electrons
Expansion of atomic radius
Weaker nuclear attraction for incoming electrons
These features combine to make electron gain progressively less favourable, diminishing oxidising strength.
Formation of 1– Ions Down the Group
The specification highlights the decreasing ease of forming 1– ions as the essential explanation for the trend.
This can be understood through the following points:
Greater atomic radius means the incoming electron is added further from the nucleus.
Increased shielding reduces effective nuclear charge.
Weaker attraction lowers the likelihood of electron capture.
Reduced electron affinity aligns with lower oxidising power.
Relationship to Chemical Behaviour
These trends in atomic structure influence observed chemical behaviour across many halogen reactions. Students should recognise that:
Chlorine reacts readily because it strongly attracts electrons.
Bromine is moderately reactive but less effective than chlorine.
Iodine has minimal tendency to accept electrons and is the weakest oxidising agent of the three.
Factors Not Responsible for the Trend
It is important to avoid misconceptions. The following do not determine the trend:
Bond enthalpies of halogen molecules are not the primary reason for reactivity changes in this subsubtopic.
Intermolecular forces affect boiling points, not electron-gain ability.
Metallic or covalent bonding concepts are unrelated to halogen oxidising power.
Summary of Trend Mechanism (Bullet Form)
Reactivity depends on how readily a halogen gains an electron.
Down the group, atomic radius increases.
Electron shielding increases, reducing effective nuclear charge.
Attraction between nucleus and incoming electron weakens.
Ease of forming 1– ions decreases, as stated by the OCR specification.
Oxidising power consequently decreases from chlorine to iodine.
FAQ
Fluorine’s extremely small atomic radius creates unusually strong repulsion between electrons in its compact 2p subshell, slightly reducing the favourability of electron gain.
Its oxidation reactions remain very rapid, but the energetics of electron acceptance are influenced by this repulsion, meaning fluorine does not always behave exactly as predicted by simple group trends.
When a halogen is reduced in water, the resulting halide ion is stabilised by solvation. The magnitude of this solvation energy affects the overall ease of electron gain.
Down the group, halide ions become larger and:
Water molecules cannot approach the ion as closely.
Solvation becomes less efficient.
Stabilisation of the halide ion is weaker.
This contributes to the reduced oxidising power of heavier halogens in aqueous solution.
Organic solvents provide weaker stabilisation of ions because they form limited ion–dipole interactions compared with water.
This means:
Electron transfer processes may become less favourable.
Halide ions are less stabilised after formation.
Displacement reactions may appear more or less vigorous than in aqueous media.
The effect is especially noticeable for iodine due to its large polarizable electron cloud.
Bond enthalpy influences the total reaction energy but is not the main cause of reactivity trends.
For example:
Chlorine has a strong Cl–Cl bond, yet still shows high oxidising power due to strong attraction for incoming electrons.
Iodine has a weaker I–I bond, but its poor ability to gain an electron dominates its behaviour.
Thus, bond enthalpies adjust—but do not dictate—the overall trend.
Displacement reactions visually confirm the trend:
Chlorine displaces bromide (orange Br2) and iodide (brown I2).
Bromine displaces iodide only, producing a brown solution.
Iodine causes no displacement.
These colour changes reflect the decreasing tendency of halogens to gain electrons down the group.
Practice Questions
Explain why chlorine is a stronger oxidising agent than iodine.
(2 marks)
Chlorine has a smaller atomic radius than iodine, so the nucleus attracts an incoming electron more strongly. (1 mark)
Chlorine has less electron shielding, making electron gain easier and increasing its oxidising power. (1 mark)
The oxidising ability of the halogens decreases from chlorine to iodine.
Using your knowledge of atomic structure, explain this trend.
In your answer, refer to atomic radius, electron shielding, effective nuclear attraction, and the formation of halide ions.
(5 marks)
Atomic radius increases down the group from chlorine to iodine. (1 mark)
Increased radius means the incoming electron is further from the nucleus. (1 mark)
Additional inner shells increase electron shielding. (1 mark)
Greater shielding reduces the effective nuclear attraction for the incoming electron. (1 mark)
As a result, forming a 1– halide ion becomes progressively more difficult, so oxidising power decreases down the group. (1 mark)

