OCR Specification focus:
‘Sulfate ions form a white precipitate with aqueous barium ions; recognition and limitations due to other insoluble salts are considered.’
Introduction
Testing for sulfate ions is an essential qualitative analysis procedure that relies on the formation of a characteristic white precipitate and requires careful control to avoid misleading results.
Test for Sulfate Ions
The test for sulfate ions (SO₄²⁻) is a key component of inorganic qualitative analysis, allowing chemists to detect the presence of sulfate-containing compounds in solution. The method relies on the formation of barium sulfate, an insoluble white precipitate, when aqueous barium ions are added under acidic conditions. This subsubtopic emphasises both the correct test procedure and the important limitations arising from other insoluble salts that may interfere with interpretation.
Essential Chemical Principle
When aqueous barium ions encounter sulfate ions in solution, they react to form barium sulfate (BaSO₄), a highly insoluble solid. This precipitate appears as a dense white solid, providing a visual confirmation of sulfate presence. To ensure validity, the reaction must occur in an acidic medium, usually achieved by adding dilute hydrochloric acid before the test reagent.
Formation of Barium Sulfate (BaSO₄) = Ba²⁺(aq) + SO₄²⁻(aq) → BaSO₄(s)
Ba²⁺ = Barium ion (aqueous)
SO₄²⁻ = Sulfate ion (aqueous)
BaSO₄(s) = Insoluble barium sulfate precipitate
The insolubility of barium sulfate is central to the reliability of the test, but other anions can also form insoluble barium salts. Understanding these limitations is an essential part of OCR A-Level Chemistry’s qualitative analysis content.
Reagents and Conditions
To perform the test correctly, two essential reagents are required:
Dilute hydrochloric acid (HCl)
Aqueous barium ions, commonly supplied as barium chloride solution (BaCl₂(aq))
The addition of dilute HCl before introducing barium ions is a crucial step that removes potential sources of false positives. The acid reacts with and removes carbonate ions, which otherwise produce a white precipitate of barium carbonate, mimicking the expected sulfate result.
Acidification: The process of adding an acid, typically dilute hydrochloric acid, to remove interfering ions before carrying out a qualitative test.
The use of hydrochloric acid is recommended because its conjugate anion, chloride, forms soluble salts with barium, preventing unwanted precipitation.
A normal sentence is placed here to maintain the required spacing before the next definition.
Barium chloride solution: A common analytical reagent providing aqueous barium ions for precipitation tests.
Step-by-Step Procedure
The recognised test sequence for sulfate ions is simple but requires strict adherence:
Procedure
Add dilute hydrochloric acid to the sample solution.
Gently swirl to ensure thorough mixing and removal of interfering ions (e.g., CO₃²⁻).
Add aqueous barium chloride dropwise.
Observe any formation of a white precipitate of barium sulfate, indicating the presence of sulfate ions.
Why Acidification Is Essential
Acidification prevents false positives caused by:
Carbonate ions (CO₃²⁻), which form barium carbonate, an insoluble white solid
Sulfite ions (SO₃²⁻), which produce barium sulfite, also a white precipitate
Both of these white precipitates could be misinterpreted as evidence for sulfate ions unless the interfering anions are removed beforehand. The added acid reacts with these ions to release gases such as carbon dioxide or sulfur dioxide, thereby eliminating them from the solution before the barium reagent is introduced.
Visual Appearance of Barium Sulfate
The characteristic white precipitate of BaSO₄ is:
Dense and finely dispersed
Persistent, not dissolving upon further acid addition
Distinct from the fizzing or gas evolution seen when interfering ions react with the acid
These features help students distinguish a genuine sulfate result from other potential observations.
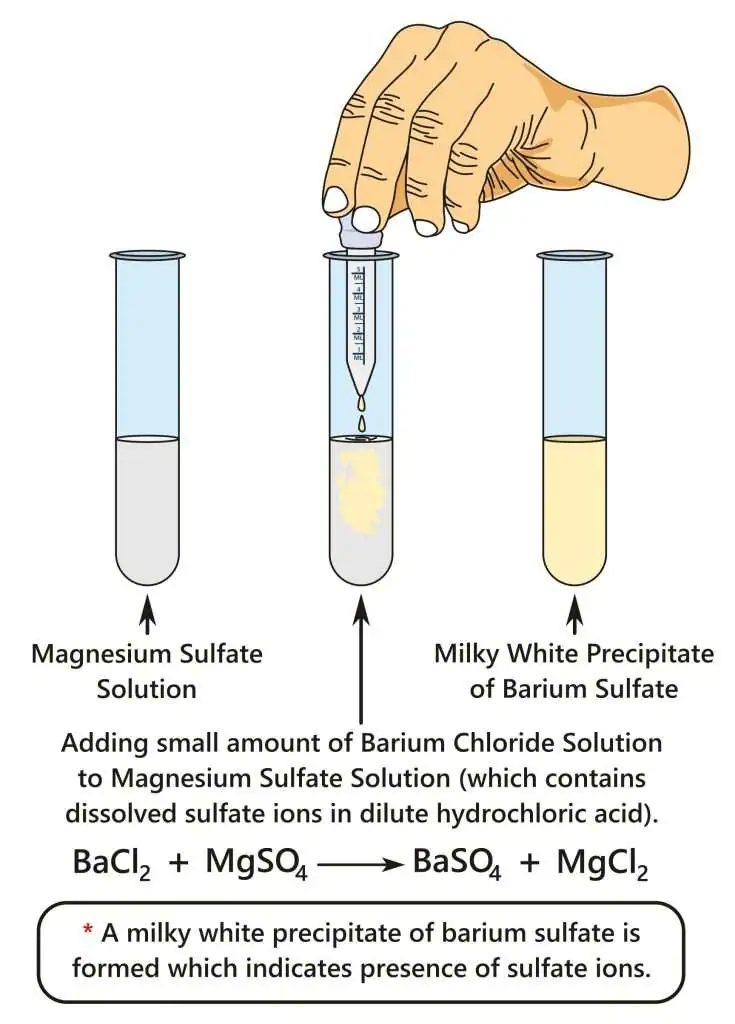
A diagram showing the reaction between sulfate ions and barium chloride solution under acidic conditions, forming a white precipitate of barium sulfate. The example uses magnesium sulfate solution, which is more specific than required but fully consistent with the OCR test. Source
Limitations of the Test
OCR emphasises the need to consider the limitations due to other insoluble salts. Although barium sulfate is diagnostic for sulfate ions, some anions form similarly insoluble barium salts:
Barium carbonate (BaCO₃)
Barium sulfite (BaSO₃)
Barium phosphate (Ba₃(PO₄)₂)
To avoid these false positives, the acidification step must not be skipped. Failure to acidify means that carbonate- or sulfite-containing samples may produce white precipitates indistinguishable from barium sulfate.
Importance of Correct Acid Choice
Hydrochloric acid is specifically used because:
Barium chloride remains soluble, preventing unwanted precipitation
Alternative acids (e.g., sulfuric acid) introduce competing anions that form insoluble salts and disrupt the test
Nitric acid may be used in other tests, but for sulfates, HCl ensures the best reliability
Choosing the correct acid is a required component of the specification and an important detail for examination questions.
Interpretation of Test Results
When carried out correctly, the presence of sulfate ions is confirmed by a white precipitate of barium sulfate that remains insoluble in excess acid. The absence of precipitate indicates that sulfate ions are not present in detectable quantities.
Key Points for OCR A-Level Students
Always acidify with dilute hydrochloric acid first.
A persistent white precipitate upon addition of barium chloride indicates SO₄²⁻ present.
Be aware of potential false positives from carbonate, sulfite, and phosphate ions.
The test depends on the low solubility of barium sulfate.

A close-up photograph of solid barium sulfate, showing its fine white powdered appearance matching the white precipitate produced during the sulfate test. Source
FAQ
Barium sulfate has an extremely low solubility in water, meaning it precipitates even when sulfate ions are present at very low concentrations.
Its stability prevents it from dissolving under acidic conditions, allowing chemists to distinguish it from other barium precipitates that may dissolve or decompose in acid.
Yes, ions such as sulfide (S²⁻) and thiosulfate (S₂O₃²⁻) can interfere, but usually under different conditions.
Sulfide ions can form barium sulfide, but they are uncommon in typical laboratory samples and produce a precipitate with a distinct smell due to hydrogen sulfide.
Thiosulfate ions can react with acid to decompose, reducing the likelihood of forming a confusing precipitate.
Barium chloride is fully soluble and provides a consistent, easily controlled source of barium ions.
Other salts such as barium nitrate could be used, but chloride ions minimise secondary precipitates because most barium chloride by-products remain soluble. This reduces the chance of obscuring or mimicking the sulfate precipitate.
Temperature has only a minor effect because barium sulfate remains highly insoluble across a wide range of temperatures.
Heating does not significantly increase its solubility, so the characteristic white precipitate forms reliably under both warm and cold conditions.
Although both appear white, their behaviour in acid differs.
Barium sulfate remains unchanged when more acid is added.
Barium carbonate reacts with acids, producing effervescence as carbon dioxide gas is released.
This reaction helps confirm whether a precipitate is due to sulfate ions or interfering carbonate ions.
Practice Questions
A student is testing an unknown solution for sulfate ions.
They add dilute hydrochloric acid to the solution, followed by a few drops of aqueous barium chloride.
(a) State the observation that would confirm the presence of sulfate ions.
(b) Explain why hydrochloric acid is added before the barium chloride solution.
(2 marks)
(a) A white precipitate forms / formation of white barium sulfate
1 mark
(b) Removes carbonate ions / prevents formation of barium carbonate, which is also a white precipitate
1 mark
A mixture containing carbonate ions and sulfate ions is tested using dilute hydrochloric acid followed by aqueous barium chloride.
(a) Describe and explain the observations you would expect when dilute hydrochloric acid is added to the mixture.
(b) After the acid is added, barium chloride solution is introduced. State the observation made and explain how this confirms the presence of sulfate ions.
(c) Explain why the test would give misleading results if the sample were not acidified first.
(5 marks)
(a)
Effervescence/fizzing observed 1 mark
Due to carbonate ions reacting with acid to produce carbon dioxide gas 1 mark
(b)
A white precipitate forms after adding barium chloride 1 mark
This precipitate is barium sulfate, which is insoluble, confirming the presence of sulfate ions 1 mark
(c)
Without acidification, carbonate ions would produce barium carbonate, a white precipitate 1 mark
This would lead to a false positive result for sulfate ions
1 mark

