OCR Specification focus:
‘Chloride, bromide and iodide give characteristic precipitates with Ag⁺(aq); their solubilities in ammonia distinguish between the halides.’
Testing halide ions involves forming distinctive silver–halide precipitates and confirming their identity using ammonia. These reactions allow reliable differentiation between chloride, bromide and iodide ions in qualitative analysis.
Testing for Halide Ions: Core Principles
Halide ions (Cl⁻, Br⁻, I⁻) are identified using silver nitrate, followed by confirmatory dissolution tests with aqueous ammonia. These steps are central to OCR A-Level qualitative analysis.
Halide identification relies on the low solubility of many silver halides, which produce characteristic colours that differ sufficiently to allow confident visual distinction. The follow-up ammonia test strengthens reliability by ensuring that similar-looking precipitates can still be differentiated.
Preparing the Test Sample
Before carrying out halide testing, the sample must be acidified using dilute nitric acid. This prevents false positives by removing interfering anions.
Interfering ion: An ion that reacts with a test reagent to give a misleading result not caused by the target ion.
Acidifying with nitric acid avoids unwanted formation of insoluble carbonates or sulfites, both of which could otherwise precipitate silver salts and compromise identification.
Why Nitric Acid Is Required
Nitric acid is used because:
It removes carbonate impurities by converting them into carbon dioxide.
It does not introduce new ions that could form precipitates with silver nitrate.
Other acids (e.g., hydrochloric acid) would introduce halide ions and invalidate the test.
Formation of Silver Halide Precipitates
Once the sample is acidified, aqueous silver nitrate (AgNO₃) is added. Each halide ion forms a distinctively coloured silver halide precipitate, allowing visual identification.
Precipitate: A solid formed in a solution as the result of a chemical reaction between aqueous ions.
Characteristic Precipitates
Chloride ions (Cl⁻): form a white precipitate of silver chloride (AgCl).
Bromide ions (Br⁻): form a cream precipitate of silver bromide (AgBr).
Iodide ions (I⁻): form a yellow precipitate of silver iodide (AgI).
Chloride, bromide and iodide ions give white, cream and yellow precipitates of silver halides when AgNO₃(aq) is added to the acidified solution.
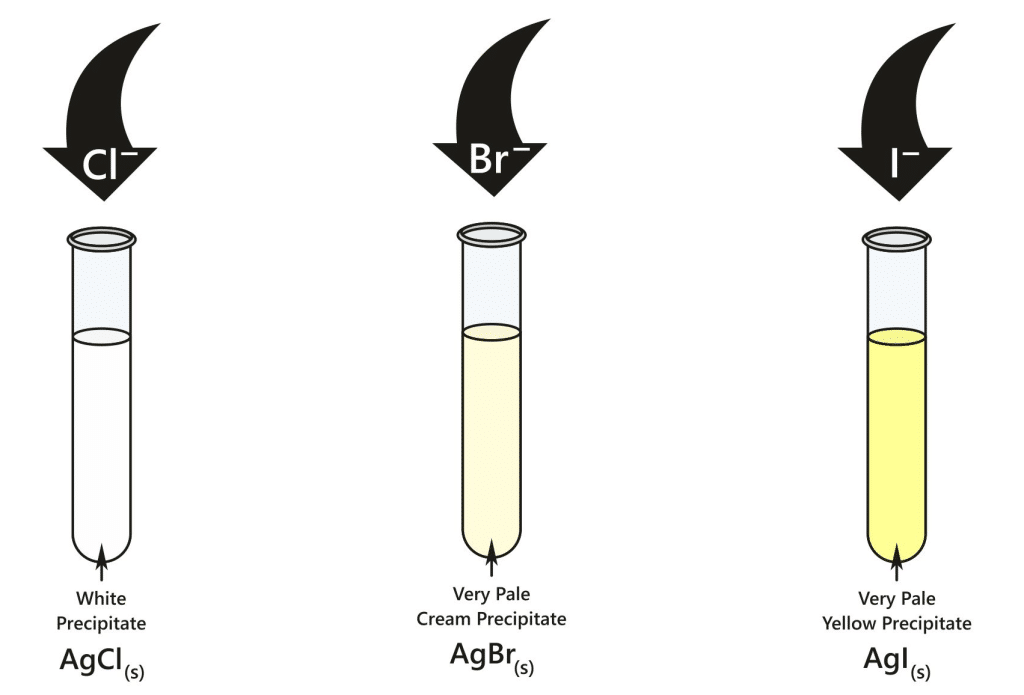
Three test tubes showing the silver halide precipitates formed with chloride (white), bromide (cream) and iodide (yellow) ions after addition of acidified silver nitrate solution. The photo illustrates the trend of increasingly deeper colour down Group 17. This image includes only the observations required by the syllabus and does not add extra content beyond the precipitate colours. Source
One difficulty students face is accurately distinguishing cream from pale yellow. For this reason, the specification requires a second confirmatory test using ammonia to eliminate ambiguity.
Ionic Equations for Precipitate Formation
Silver halide formation (AgX) = Ag⁺(aq) + X⁻(aq) → AgX(s)
Ag⁺ = silver ion (no unit)
X⁻ = halide ion (no unit)
These equations, while simple, emphasise the direct 1:1 reaction typical of halide precipitation processes.
Confirming Halides Using Aqueous Ammonia
The second stage involves adding dilute aqueous ammonia, then concentrated aqueous ammonia if required. Differences in silver halide solubility produce a predictable pattern used to confirm halide identity.
The dissolution behaviour depends on the formation of complex ions, which increases silver ion solubility for some halides.
Behaviour of Silver Halides with Ammonia
Silver chloride (AgCl):
Dissolves in dilute ammonia, producing a colourless solution.
Silver bromide (AgBr):
Insoluble in dilute ammonia, but dissolves in concentrated ammonia.
Silver iodide (AgI):
Insoluble in both dilute and concentrated ammonia, remaining yellow.
Adding dilute then concentrated ammonia distinguishes the halides further because the silver halide precipitates have different solubilities.
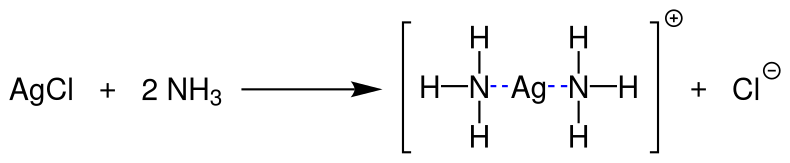
Equilibrium diagram showing solid silver chloride reacting with aqueous ammonia to form the soluble complex ion [Ag(NH₃)₂]⁺ and chloride ions. This illustrates why the white AgCl precipitate dissolves in dilute ammonia, whereas other silver halides are less soluble. The diagram focuses on AgCl only and does not show the behaviour of AgBr or AgI, which must be inferred separately from the study notes. Source
Underlying Chemistry: Formation of Complex Ions
When soluble, silver halides interact with ammonia to form the diamminesilver(I) complex, which is highly soluble.
Silver–ammonia complex formation ([Ag(NH₃)₂]⁺) = AgCl(s) + 2NH₃(aq) → [Ag(NH₃)₂]⁺(aq) + Cl⁻(aq)
Ag⁺ = silver ion (no unit)
NH₃ = ammonia (no unit)
[Ag(NH₃)₂]⁺ = diamminesilver(I) complex ion (no unit)
This equation demonstrates why AgCl and AgBr behave differently: the strength of Ag–X bonding decreases AgX solubility down the group.
Practical Procedure for Testing Halide Ions
To ensure clarity and consistency, the OCR specification expects students to apply the correct test sequence and reagent additions.
Step-by-Step Method
Step 1: Add dilute nitric acid to the sample to remove interfering ions.
Step 2: Add aqueous silver nitrate and observe precipitate colour.
Step 3: Add dilute ammonia and record any dissolution.
Step 4: If still undissolved, add concentrated ammonia and observe further changes.
This sequence reduces risk of misidentification and ensures compliance with standard qualitative analysis protocols.
Key Observations to Record
Students should pay close attention to:
Initial precipitate colour (white, cream, or yellow).
Effect of dilute ammonia (dissolves AgCl only).
Effect of concentrated ammonia (dissolves AgBr but never AgI).
Whether any precipitate remains, confirming iodide.
Analytical Considerations and Good Laboratory Practice
Careful observation is critical because lighting conditions can alter perceived colours. Recording results immediately prevents confusion, especially when comparing cream and yellow precipitates. Additionally, clean glassware must be used, as residual ions can distort results.
Acidification should always precede silver nitrate addition; otherwise, unwanted precipitates such as Ag₂CO₃ may form, giving a false suggestion of halides. Awareness of these potential errors increases student reliability when interpreting test results.
Finally, the halide test plays a central role in multi-stage qualitative analysis, and its consistent structure—precipitation followed by selective dissolution—makes it robust, predictable and highly examinable within OCR A-Level Chemistry.
FAQ
Nitric acid is used because it avoids introducing additional ions that could produce misleading precipitates. Hydrochloric and hydrobromic acids contain halide ions, while sulfuric acid introduces sulfate ions capable of forming insoluble salts with silver ions.
Additionally, nitric acid provides a strongly acidic environment that removes carbonate or sulfite impurities without creating new precipitates that interfere with halide identification.
Silver bromide appears cream, whereas silver iodide is a deeper yellow, but lighting conditions may obscure the difference. The most reliable approach is to use ammonia:
AgBr dissolves in concentrated ammonia
AgI does not dissolve in either dilute or concentrated ammonia
This method eliminates ambiguity caused by subjective colour perception.
Their differing solubilities arise from variations in the strength of the silver–halide bond and the ease with which the silver ion forms a complex with ammonia.
Down the group, the halide ions become larger and less polarising, leading to stronger lattice enthalpies in AgX. The stronger the lattice enthalpy, the less easily the precipitate dissolves, making AgI the least soluble.
Silver nitrate can stain skin and clothing and should be handled carefully to avoid spills. It is also an oxidising agent, requiring safe storage and eye protection.
Ammonia produces a pungent, irritating vapour. Work in a well-ventilated area or fume cupboard, and avoid inhaling fumes. Ensure containers are sealed when not in use.
Carbonate and sulfate ions can both produce precipitates with silver nitrate, leading to false positives if the halide test is done too early.
Performing the tests in the correct order ensures:
Carbonates are removed before sulfate testing
Sulfates are removed before halide testing
Only genuine silver halide precipitates remain, providing reliable identification
Practice Questions
A student adds dilute nitric acid followed by aqueous silver nitrate to an unknown solution. A cream precipitate forms.
(a) Identify the halide ion present.
(b) State the observation when dilute ammonia is added to the precipitate.
(2 marks)
(a) Bromide ion / Br–
Correct identification of bromide (1 mark)
(b) Cream precipitate does not dissolve in dilute ammonia
Correct observation (1 mark)
Total: 2 marks
A sample is suspected to contain a mixture of halide ions.
(a) Explain why dilute nitric acid must be added before testing the sample with silver nitrate.
(b) Describe how aqueous ammonia can be used to distinguish between chloride, bromide and iodide ions once the silver halide precipitates have formed.
(c) A student reports a pale yellow precipitate that does not dissolve in either dilute or concentrated ammonia. Identify the halide ion present and justify your answer.
(5 marks)
(a)
Nitric acid removes carbonate or other interfering ions (1 mark)
Prevents formation of unwanted silver precipitates that could give false positives (1 mark)
(b)
Silver chloride dissolves in dilute ammonia (1 mark)
Silver bromide dissolves only in concentrated ammonia (1 mark)
Silver iodide does not dissolve in either dilute or concentrated ammonia (1 mark)
(c)
Iodide ion / I– identified (1 mark)
Justification: AgI is insoluble in both dilute and concentrated ammonia (1 mark)
Total: 5 marks

