OCR Specification focus:
‘Ammonium ions release ammonia on warming with aqueous sodium hydroxide; the alkaline gas is identified by its characteristic effect on indicators.’
Aqueous ammonium ions are detected through their reaction with warm sodium hydroxide, producing ammonia gas. Clear observations and reliable confirmatory steps ensure accurate qualitative analysis.
The Chemical Basis of the Ammonium Ion Test
The ammonium ion, NH₄⁺, behaves as a weak acid in solution and releases ammonia gas (NH₃) when heated with aqueous hydroxide ions. This reaction forms the basis of the test. Ammonia’s distinct alkaline properties make it easily identifiable using moist indicator paper.
Ammonia (NH₃): A pungent, alkaline gas produced when ammonium ions are warmed with hydroxide ions.
Ammonia’s high solubility in water and its ability to turn damp indicators blue provide simple yet conclusive evidence of ammonium ions in a sample.
Reagents and Apparatus Required
The test relies on basic laboratory reagents that allow both the release and confirmation of ammonia gas.
Essential reagents and equipment include:
Aqueous sodium hydroxide, providing hydroxide ions for the reaction
A test tube and heat source
Damp red litmus paper or moist universal indicator paper for detecting alkaline gases
A glass rod for handling indicator paper safely
Moist indicator paper must always be used so that gaseous ammonia dissolves on its surface and undergoes the observable colour change associated with alkaline conditions.
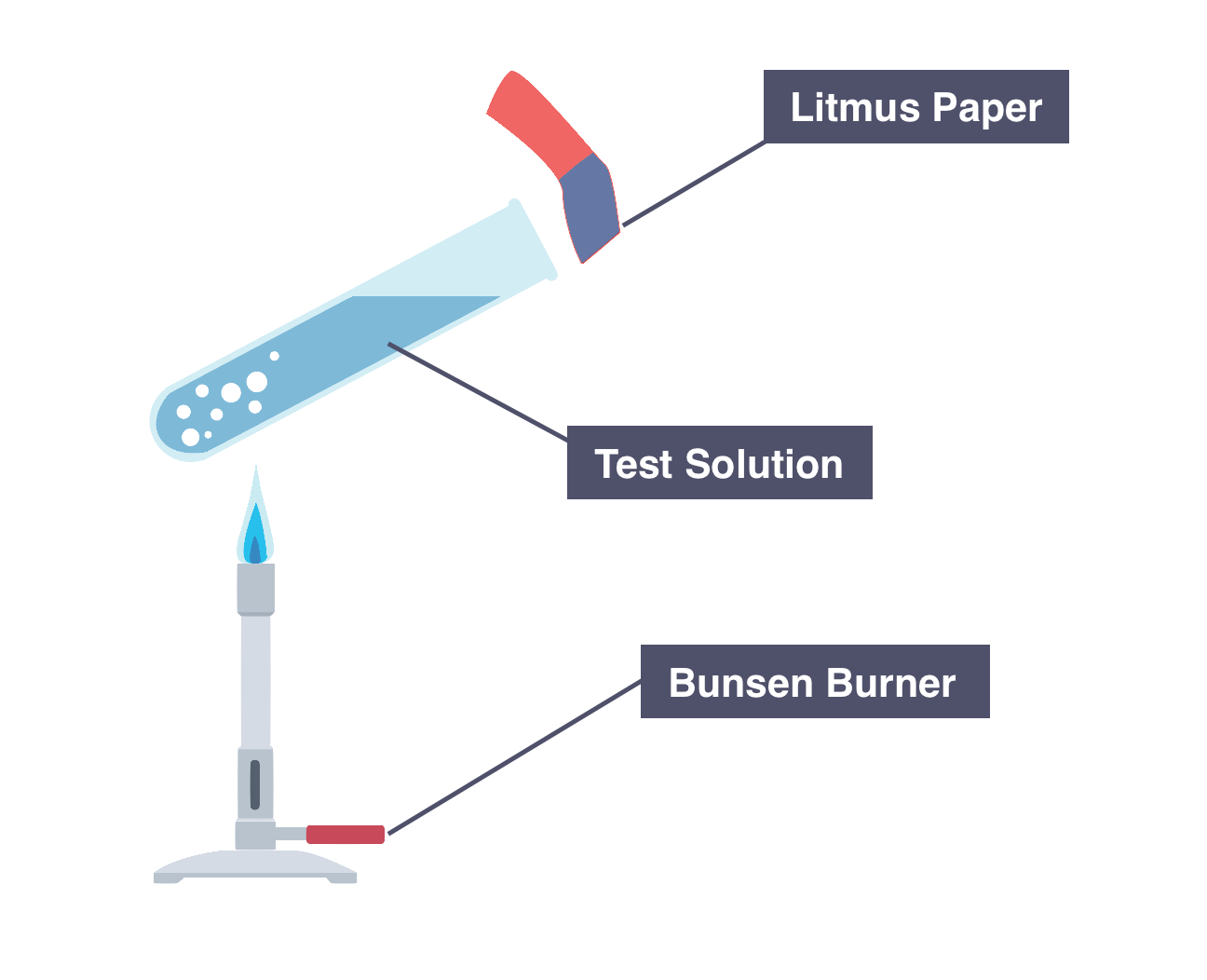
Diagram of the standard laboratory setup for testing for ammonium ions with aqueous sodium hydroxide. The test solution is warmed while damp litmus paper is held at the mouth of the tube to detect evolved ammonia. This also reinforces safe positioning of the indicator outside the solution to prevent contamination. Source
The Test Procedure
Step-by-Step Method
Students must understand not only the observations but also why each step is necessary. The procedure should be followed in a controlled manner to avoid loss of gas before detection.
To carry out the ammonium ion test:
Add a small volume of aqueous sodium hydroxide to the test sample.
Gently warm the mixture; avoid boiling, which may expel the gas too rapidly.
Hold damp red litmus paper or moist universal indicator paper at the mouth of the test tube.
Observe any colour change of the indicator.
Note the presence of a distinctive pungent smell, characteristic of ammonia.
Aqueous sodium hydroxide is essential because it provides excess hydroxide ions that shift the equilibrium toward ammonia formation, ensuring detectable gas release.
Chemical Reaction Occurring
The process can be represented by a simple ionic equation illustrating ammonia liberation.
Ammonium Ion Decomposition: NH₄⁺(aq) + OH⁻(aq) → NH₃(g) + H₂O(l)
NH₄⁺ = Ammonium ion in solution
OH⁻ = Hydroxide ion provided by sodium hydroxide
NH₃ = Ammonia gas released on warming
The ionic equation demonstrates that heating is not creating new ions but merely driving a shift in equilibrium to favour gaseous ammonia.
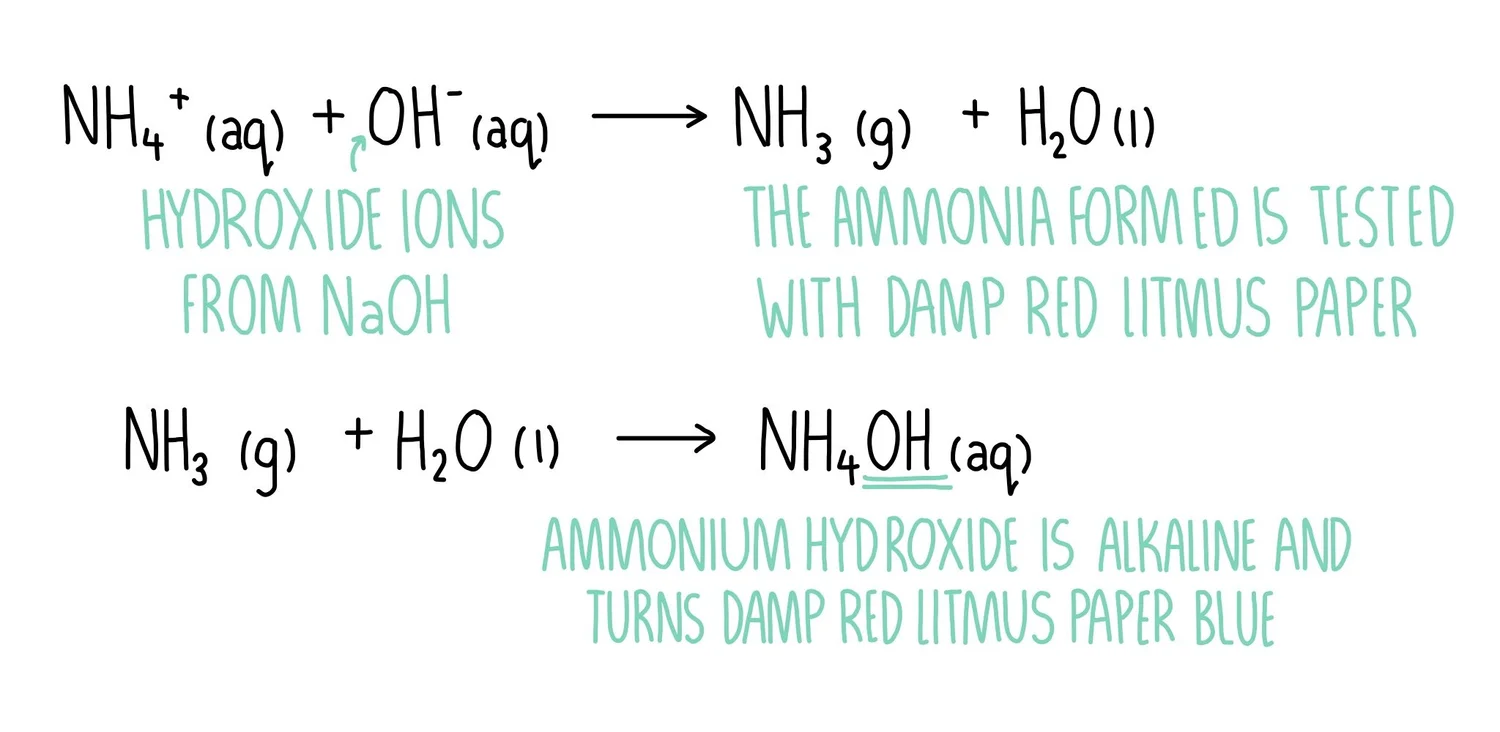
Annotated equations showing how ammonium ions react with hydroxide ions to release ammonia gas, followed by its dissolution to form alkaline ammonium hydroxide. This explains the blue colour change in damp red litmus paper. The inclusion of ammonium hydroxide extends beyond the strict OCR wording but clarifies the observed alkalinity. Source
Key Observations and Confirmatory Evidence
Indicator-Based Identification
Ammonia is a soluble alkaline gas, meaning it dissolves in the moisture of indicator paper, causing an immediate colour change to blue. This is the key confirmatory observation required by the specification.
Characteristic observations include:
Damp red litmus paper turns blue
Moist universal indicator turns blue or purple, depending on the concentration of ammonia
Sharp, pungent odour typical of ammonia
These observations differentiate ammonia from neutral or acidic gases, giving the ammonium ion test high reliability.
Why the Indicator Must Be Damp
Because ammonia is highly soluble, especially in water, it must interact with moisture to produce the alkaline solution necessary for the colour change. Dry indicator paper may fail to show any result because the gas may not dissolve sufficiently to ionise and trigger the visible shift.
A damp surface allows ammonia to react, forming ammonium and hydroxide ions locally, which then cause the alkaline indicator response.
Avoiding False Positives and Practical Considerations
Importance of Heating Gently
Overheating may disperse ammonia too quickly for effective detection, while insufficient warming may produce only minimal gas, leading to weak or missed observations.
Avoiding Contamination
Glassware should be washed thoroughly to prevent misleading results, especially because traces of cleaning agents or previous reagents may produce alkaline fumes. Sodium hydroxide pellets or concentrated solutions must never be used directly for the indicator test because they can cause the litmus paper to turn blue independently of any ammonia.
Handling Airborne Ammonia
Ammonia is volatile and disperses rapidly. Students should ensure:
The indicator is positioned at the mouth of the tube, not inside it
The test is performed away from other open alkaline reagents
Personal safety is maintained, as ammonia gas can irritate the respiratory system
These checks ensure accurate interpretation of the test outcome and reinforce safe laboratory practice.
Linking Observations to Ammonium Ion Presence
When ammonia gas is detected, it serves as direct confirmation that the original sample contained ammonium ions. No other common ions in qualitative analysis release ammonia under these standard test conditions, making the result definitive when the procedure is correctly followed.
Because this test is included in the qualitative analysis sequence, it is typically performed after carbonate, sulfate, and halide tests. However, for the ammonium ion test itself, only the correct identification of ammonia gas matters for confirming NH₄⁺.
This structured approach ensures students can reliably detect ammonium ions using simple reagents, clear observations, and a sound understanding of the underlying chemistry.
FAQ
Ammonia is uniquely identified by its combination of a pungent, sharp smell and its ability to turn damp red litmus paper blue almost instantly.
Other alkaline gases are far less common in standard inorganic qualitative analysis. Ammonia also forms dense white fumes when exposed to hydrogen chloride gas, providing an additional distinguishing feature not shared by most alkaline vapours.
Gentle heating releases ammonia steadily, allowing controlled detection at the mouth of the test tube.
Strong heating may cause ammonia to disperse too rapidly for reliable testing, and it can also lead to bumping or loss of sample, reducing test accuracy.
Ammonia is irritating to the eyes and respiratory system. Students should avoid inhaling the gas directly and should keep the test tube pointed away from themselves and others.
Additional precautions include:
Working in a well-ventilated area
Keeping indicator paper outside the test tube
Avoiding contact of sodium hydroxide with skin or eyes
Sodium hydroxide provides a high concentration of hydroxide ions, ensuring efficient displacement of ammonia from the ammonium ion.
Other bases may be too weak or insufficiently soluble to shift the equilibrium fully. Sodium hydroxide also remains chemically inert to most other species in qualitative analysis, minimising side reactions.
Certain impurities may produce alkaline fumes or contain residual cleaning agents, causing false positives.
To minimise interference:
Ensure glassware is thoroughly rinsed
Avoid using containers previously exposed to strong bases
Confirm suspicious results by repeating the test with a freshly prepared portion of the sample
Practice Questions
A student warms an aqueous solution with sodium hydroxide and holds a piece of damp red litmus paper at the mouth of the test tube.
The litmus paper turns blue.
(a) State the ion present in the original solution.
(b) Explain why the litmus paper must be damp.
(2 marks)
(a) Ammonium ion / NH4+
1 mark for correctly identifying ammonium ions.
(b) The litmus must be damp so ammonia can dissolve and produce an alkaline solution.
1 mark for explaining that moisture is needed for the colour change.
A solid sample is suspected to contain ammonium ions.
Describe the full practical test the student should carry out to confirm the presence of ammonium ions.
In your answer, include:
the reagents used
the observations expected
the chemical equation for the reaction
an explanation for how the observation confirms the presence of ammonium ions.
(5 marks)
Award marks for the following points:
Add aqueous sodium hydroxide to the solid or its solution. (1 mark)
Warm the mixture gently. (1 mark)
Test the gas at the mouth of the test tube using damp red litmus paper. (1 mark)
Observation: litmus paper turns blue (or a pungent smell is detected). (1 mark)
Correct ionic equation: NH4+(aq) + OH–(aq) → NH3(g) + H2O(l). (1 mark)
Explanation: ammonia is alkaline, so the colour change confirms ammonium ions were present. (Accept: only ammonium ions release ammonia under these conditions.) (1 mark)
Maximum 5 marks.

