OCR Specification focus:
‘Carry out tests in sequence: carbonate, sulfate, then halide, to avoid insoluble precipitate interferences such as BaCO₃ and Ag₂SO₄.’
Understanding the correct analytical test sequence is essential in qualitative inorganic analysis, ensuring reliable results by preventing contaminating precipitates and avoiding false positives that arise from incompatible reagent interactions.
The Need for a Structured Test Sequence
In qualitative analysis, the order in which tests are conducted is crucial for obtaining accurate results. The OCR specification emphasises a fixed sequence—carbonate, then sulfate, then halide—because many ions form insoluble precipitates when reagents overlap. Without following a structured order, students may unknowingly introduce ions that interfere with later tests, leading to incorrect conclusions about an unknown sample’s composition.
Why Test Carbonates First
Carbonate ions react readily with acids to release carbon dioxide gas.
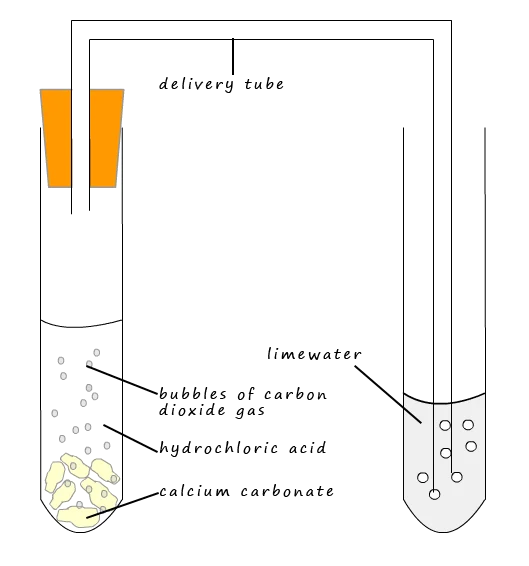
A diagram of the carbonate ion test showing calcium carbonate reacting with dilute hydrochloric acid to release carbon dioxide. The gas is passed through limewater, which turns cloudy if CO₂ is present. The limewater confirmatory step is additional detail beyond the 5.4.1 scope but supports understanding of why carbonates are tested first. Source
Carbonate Ion Test: The detection of carbonate ions using a dilute acid to release carbon dioxide gas, which is then identified through characteristic observations.
Introducing an acid at a later stage would dissolve or modify precipitates formed in earlier tests, obscuring results. The carbonate test is therefore always performed first to remove ambiguity and confirm whether CO₃²⁻ is present before any other reagents are added.
Key reasons for positioning the carbonate test first:
Acids interfere with many subsequent precipitation reactions.
Carbonate-containing samples would otherwise disrupt later stages by forming unwanted gaseous or solid by-products.
Identifying carbonates early prevents misinterpretation of later precipitates.
Rationale for Testing Sulfates After Carbonates
Once carbonates are eliminated or confirmed, the next step is to test for sulfate ions. This relies on adding a soluble barium salt to produce a white precipitate of barium sulfate if sulfate ions are present.
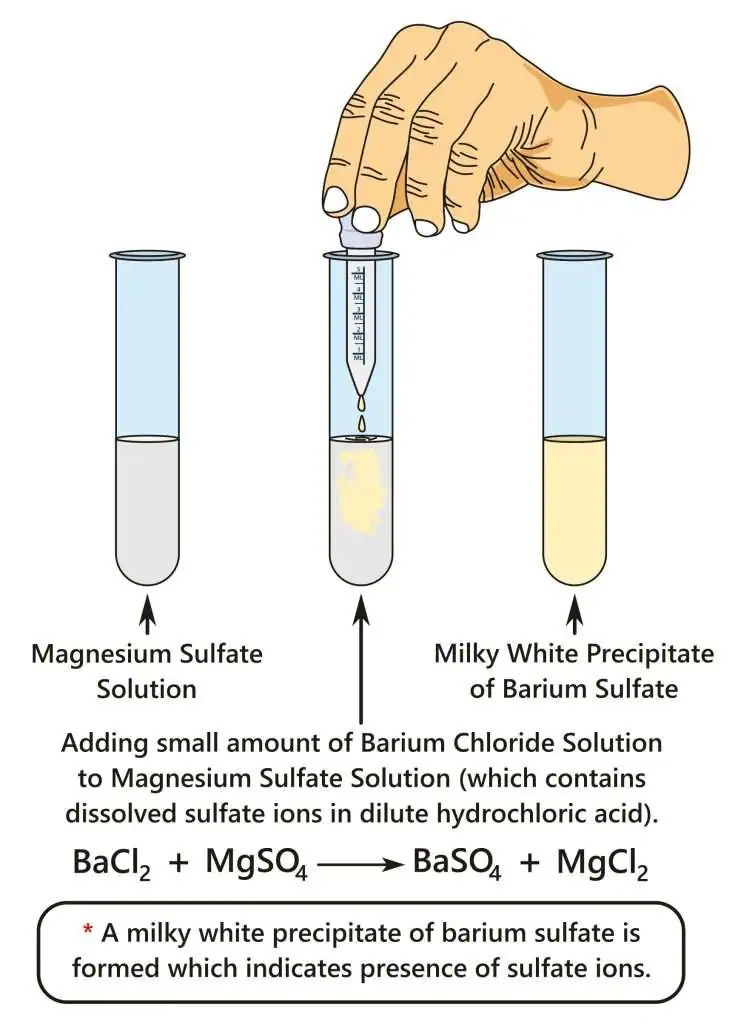
Illustration of the sulfate ion test, where dilute barium chloride solution is added to an acidified magnesium sulfate solution. A milky white precipitate of barium sulfate forms if sulfate ions are present. The use of hydrochloric acid shown here aligns with the need to remove carbonate impurities before the sulfate test. Source
Sulfate Ion Test: The identification of sulfate ions using aqueous barium ions to form a white precipitate of barium sulfate, indicating the presence of SO₄²⁻.
A normal sentence must follow to maintain the required spacing and clarity.
If sulfate testing were performed after the halide test, unwanted precipitates such as BaCO₃ could appear when carbonate impurities remain, or reagents may have been removed through earlier steps.
Key reasons to position the sulfate test second:
Prevents formation of insoluble barium carbonate, which would appear if carbonates were not excluded beforehand.
Avoids the risk of confusing BaSO₄ with other barium salts.
Ensures barium-based testing is conducted before silver nitrate is introduced.
Why Halides Are Tested Last
The halide test involves using silver nitrate to form characteristic silver halide precipitates. These precipitates—AgCl, AgBr, and AgI—differ in colour and solubility, enabling reliable identification when the test is performed correctly and in the correct order.
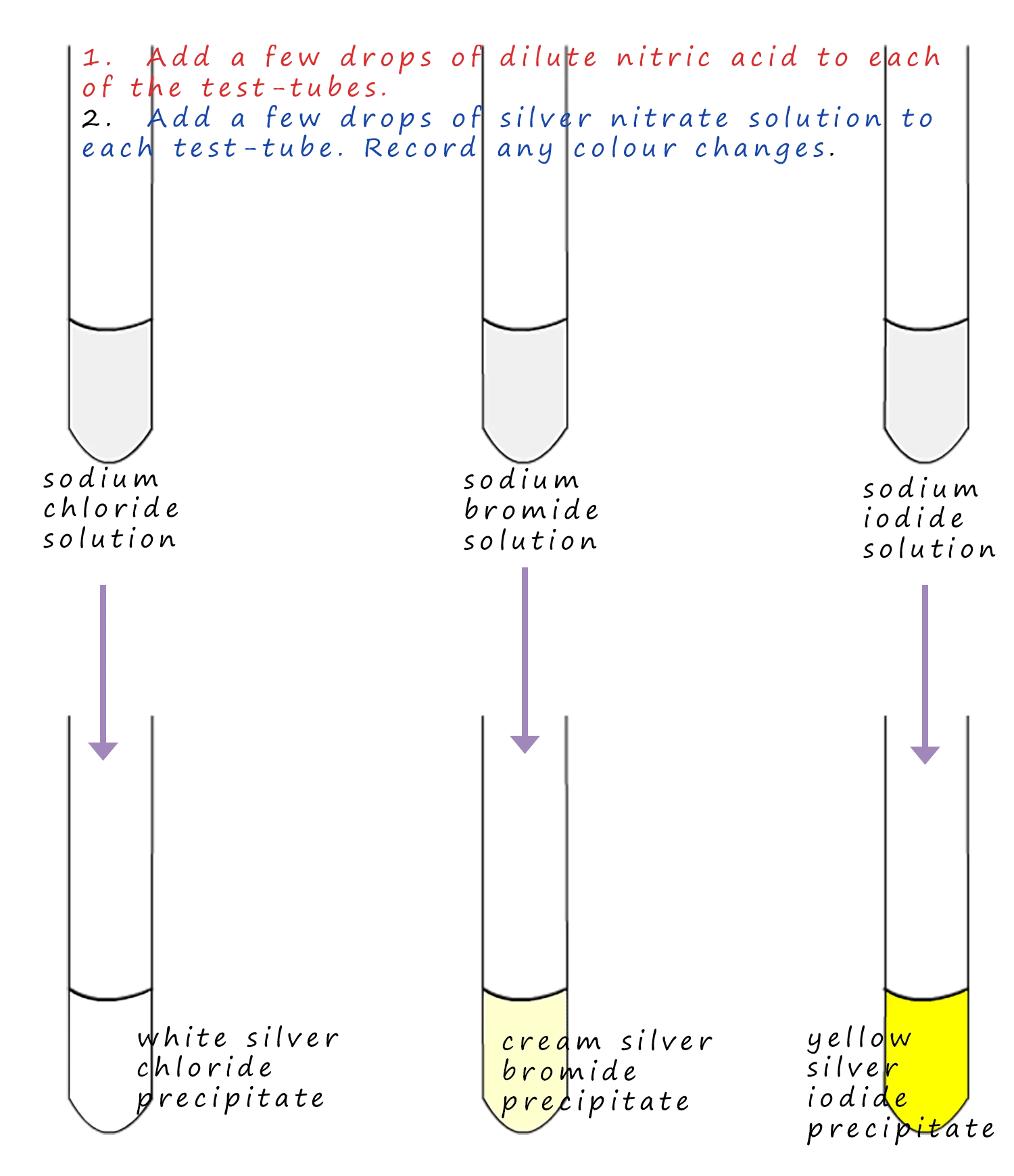
A schematic of the halide ion test using silver nitrate solution. Sodium chloride, bromide and iodide solutions give white, cream and yellow silver halide precipitates respectively. The diagram focuses on precipitate colours; confirmatory ammonia steps mentioned in the notes are not shown. Source
Halide Ion Test: The detection of halide ions using aqueous silver ions to form characteristic silver halide precipitates, followed by ammonia to distinguish between them.
Silver nitrate is highly reactive with other anions, especially sulfates and carbonates, which can form Ag₂SO₄ or Ag₂CO₃—both unwanted and misleading. Ensuring that carbonate and sulfate ions have already been removed or confirmed prevents the introduction of these problematic precipitates.
Reasons halides must be tested last:
Silver ions react with many anions, so earlier tests must remove interfering species.
Avoids formation of incorrect precipitates such as Ag₂SO₄.
Prevents silver reagents from disturbing barium-based tests if used earlier.
Interferences and Their Chemical Origins
Understanding why interferences occur is essential for applying the test sequence correctly. Many interfering species form insoluble ionic lattices, causing unintended precipitates that obscure results. These include:
Barium carbonate (BaCO₃): Forms if carbonate ions are still present when barium reagents are added.
Silver sulfate (Ag₂SO₄): Forms if sulfate ions remain when silver nitrate is added.
Silver carbonate (Ag₂CO₃): Forms when silver nitrate meets carbonate ions.
These precipitates share similar colours and appearances with the desired testing products, increasing the risk of misidentification.
The Logical Flow of Reagent Aggressiveness
The required order also reflects the reactivity strength of the reagents involved:
Acidic reagents in the carbonate test have immediate, broad-spectrum effects and must be used first.
Barium reagents selectively target sulfate ions but will also react with carbonates if present.
Silver ions (from silver nitrate) react with the greatest number of anions, so they are used last to preserve clarity.
By following this flow—from the most reactive/nonspecific reagent to the most selective—the analysis remains controlled, reliable, and unambiguous.
Practical Notes for Applying the Sequence
To support the required test order, students should implement the following practices:
General procedural reminders
Use separate test tubes for each stage to avoid contamination.
Always add reagents in small, incremental amounts to monitor precipitate formation clearly.
Record all observations immediately, noting colour, solubility changes, and any effervescence.
Correct reagent use
For carbonates: use a dilute acid, observing gas formation.
For sulfates: use aqueous barium ions, often from barium chloride or barium nitrate.
For halides: use silver nitrate, followed by ammonia for confirmatory testing.
Maintaining sample integrity
Ensure no reagent excess carries over between stages.
Avoid unnecessary heating or mixing before confirming earlier test results.
Recognise that reagent order safeguards reliability by preventing misleading precipitates.
Adhering to the carbonate → sulfate → halide sequence ensures that each test is chemically isolated, preventing cross-contamination and maintaining the validity of qualitative analysis.
FAQ
Acidifying removes carbonate ions that could otherwise produce misleading precipitates such as barium carbonate or silver carbonate.
Dilute acids also prevent metal ions from forming hydroxides with traces of alkali in the solution, keeping results consistent.
Only non-interfering acids such as hydrochloric or nitric acid are used to avoid introducing new anions that could affect the outcome.
Use clean test tubes for each stage to avoid cross-contamination.
Do not reuse pipettes without rinsing thoroughly, as leftover ions can trigger unwanted precipitation.
If solids form during testing, decanting rather than filtering prevents loss of sample that may be needed for subsequent tests.
Excessively concentrated reagents may produce precipitates too rapidly to observe accurately, making it difficult to distinguish subtle colour differences.
Dilute reagents allow gradual formation of precipitates, helping students assess solubility, texture, and rate of appearance.
Using consistent concentrations also ensures comparison between samples is reliable and reproducible.
Heating may cause decomposition of some ionic compounds, releasing gases or forming alternative solid products that mimic genuine test outcomes.
Temperature changes also affect solubility, sometimes causing precipitates to dissolve or form unexpectedly.
Keeping the sample at room temperature ensures that results arise from the intended chemical reactions only.
Check that all reagents are fresh, as degraded solutions may not provide sufficient ions for precipitation.
Ensure sufficient sample volume is present; very dilute solutions may produce minimal visible precipitate.
If still unclear, repeat the test on a more concentrated sample while keeping the same reagent order to avoid interference.
Practice Questions
Explain why the carbonate test must be carried out before the sulfate and halide tests in qualitative analysis.
(2 marks)
1 mark: States that carbonates react with acids to produce carbon dioxide gas, which would interfere with later tests if not identified first.
1 mark: Recognises that acids used later would affect or dissolve precipitates formed in the sulfate or halide tests, giving misleading results if the carbonate test were not done first.
A student analyses an unknown solution following the correct sequence of qualitative tests: carbonate, then sulfate, then halide.
Describe the reasoning behind this required order and explain what could go wrong if the tests were performed in a different sequence. Refer to specific interfering precipitates in your answer.
(5 marks)
1 mark: States that the carbonate test must be performed first because acids used in this test would dissolve or disrupt precipitates formed in later tests.
1 mark: States that the sulfate test must be carried out second because barium ions would otherwise react with carbonate ions to produce insoluble barium carbonate.
1 mark: States that halides are tested last because silver ions react with many anions, including carbonates and sulfates, forming unwanted precipitates.
1 mark: Identifies at least one incorrect precipitate that could form, such as BaCO3, Ag2SO4 or Ag2CO3.
1 mark: Provides a clear explanation of how performing the tests out of order could lead to false positives or misidentification of ions.

