OCR Specification focus:
‘Catalysts increase rate without being used up by providing routes with lower activation energy, shown on enthalpy profiles; homogeneous and heterogeneous types defined.’
Catalysts play a central role in chemical kinetics by offering alternative reaction pathways that require less energy, significantly influencing reaction rates and industrial processes.
Understanding Catalysts
Catalysts are substances that alter the rate of a chemical reaction while remaining chemically unchanged at the end. When a catalyst is first introduced in this context, it is essential to define it precisely.
Catalyst: A substance that increases the rate of a chemical reaction without being used up, by providing an alternative pathway with lower activation energy.
A catalyst does not affect the overall enthalpy change of a reaction, nor does it alter the position of equilibrium. Instead, it influences the kinetics of the process, making reactions proceed faster under the same conditions.
Alternative Reaction Pathways
A key feature of catalytic action is the provision of an alternative reaction pathway. This pathway has a lower activation energy, enabling a greater proportion of reacting particles to reach the energy threshold needed to form products.
Catalysts reduce the height of the activation energy barrier, as shown clearly on enthalpy profile diagrams.
Lower activation energy means a higher fraction of molecules possess sufficient energy to react at a given temperature.
The overall enthalpy change (ΔH) remains unchanged because reactants and products are unaffected thermodynamically.
Activation energy: The minimum energy required for a reaction to take place when particles collide.
Catalysts therefore change the rate rather than the energetics of a reaction.
On an enthalpy profile diagram, a catalyst provides an alternative route with a lower activation energy while the overall enthalpy change (ΔH) between reactants and products stays the same.
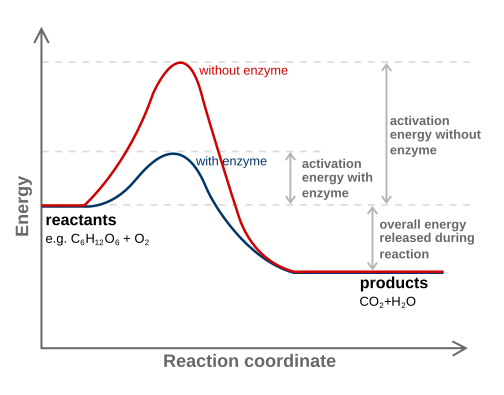
Energy profile for a reaction comparing uncatalysed and catalysed pathways. The catalysed route has a noticeably lower activation energy peak, but the reactants and products lie at the same energy, so ΔH is unchanged. This visual emphasises that catalysts speed up reactions by lowering Ea, not by altering the overall enthalpy change. Source
Enthalpy Profile Diagrams and Catalysts
Enthalpy profile diagrams illustrate the change in enthalpy as a reaction progresses from reactants to products. When a catalyst is present:
The diagram includes a lower peak, representing the reduced activation energy.
The relative enthalpies of reactants and products remain the same.
This visual comparison helps clarify why catalysed reactions proceed more rapidly.
Such diagrams are a crucial part of OCR A-Level Chemistry and are widely used to explain catalytic behaviour.
Types of Catalysts
Catalysts are commonly classified based on the physical state of the catalyst relative to the reactants. The specification emphasises the distinction between homogeneous and heterogeneous catalysts.
Homogeneous Catalysts
A homogeneous catalyst is in the same physical state as the reactants, usually aqueous or gaseous.
Homogeneous catalyst: A catalyst that is in the same physical state as the reactants in the reaction mixture.
Homogeneous catalysis typically involves the catalyst forming an intermediate species with the reactants. This intermediate then decomposes to regenerate the catalyst and produce the reaction products.
Important features include:
Close molecular interaction with reactants
Often used in biochemical and solution-based reactions
Catalyst and reactants mix uniformly, promoting effective collisions
Homogeneous catalysts are advantageous where precise control over reaction mechanisms is required.
Heterogeneous Catalysts
In heterogeneous catalysis, the catalyst is in a different physical state from the reactants, usually a solid with gases or liquids reacting at its surface.
Schematic of a heterogeneous catalytic system showing reactant molecules interacting with the surface of a solid catalyst in a separate phase. The diagram highlights how reactions occur at the interface, with adsorption, reaction, and product release. Any additional industrial context shown goes beyond the OCR syllabus but still illustrates the same key idea: heterogeneous catalysts operate at a surface between phases. Source
Heterogeneous catalyst: A catalyst that is in a different physical state from the reactants, typically a solid catalyst with gaseous reactants.
Heterogeneous catalysis involves several essential steps:
Adsorption of reactant molecules onto the catalyst surface
Weakening of bonds within adsorbed molecules
Reaction on the catalyst surface
Desorption of product molecules, leaving the catalyst available for reuse
Key features include:
Catalyst surfaces contain active sites crucial for reaction
Common in industrial processes such as hydrogenation and catalytic converters
Easy separation of catalyst and products, improving practicality
Heterogeneous catalysts are widely used because they offer durability, reusability, and straightforward recovery.
How Catalysts Affect Reaction Rate
Catalysts influence reaction rate by increasing the number of successful collisions per unit time. They do so by:
Lowering activation energy, increasing the fraction of molecules that exceed this threshold
Providing specific binding sites that stabilise transition states
Enhancing the orientation or local concentration of reactants
These effects are directly linked to the ideas of collision theory and energy distribution, although those topics belong to separate subsubtopics.
Rate (r) = Change in concentration / Time
Concentration = Amount of substance per unit volume (mol dm⁻³)
Time = Duration over which the concentration change occurs (s)
Although this relationship does not change with the presence of a catalyst, the observed rate increases because the catalyst allows more successful collisions.
A catalyst’s effectiveness depends on factors such as surface area (for heterogeneous systems), catalyst purity, and temperature.
Catalyst Behaviour in Reactions
Catalysts must be regenerated at the end of a reaction. Their role is cyclical:
Participate in the reaction mechanism
Form intermediates
Re-emerge unchanged after product formation
Important considerations:
The catalyst must be chemically stable
Poisons can block active sites in heterogeneous catalysts, reducing efficiency
Reaction conditions must be compatible with catalyst integrity
Catalysts are essential for modern chemical synthesis and environmental technologies, aligning with the specification’s emphasis on alternative pathways and activation energy reduction.
FAQ
Catalysts may lose effectiveness due to poisoning, in which impurities bind to active sites and prevent reactant adsorption. This does not consume the catalyst chemically but blocks its function.
Surface catalysts may also suffer from sintering at high temperatures, causing active sites to merge and reduce overall surface area.
In heterogeneous systems, product molecules can occasionally remain adsorbed, temporarily inhibiting activity until conditions remove them.
Catalyst structure determines how effectively reactants adsorb, orient, and transition to products.
Key structural features include:
Active sites that stabilise transition states
Surface shape and geometry, influencing adsorption strength
Presence of promoters that modify electronic properties of the surface
These characteristics allow catalysts to form lower-energy intermediates, reducing the energy required for the reaction to proceed.
Heterogeneous catalysts are easier to separate from reaction mixtures, reducing purification costs.
They are also more robust under extreme temperatures and pressures, making them suitable for large-scale continuous processes.
Additionally, surface-based reactions allow for controlled activity, as catalyst particle size, support material, and active site density can be engineered precisely.
A catalyst offers an alternate sequence of elementary steps, each with lower individual activation energies than the uncatalysed route.
Although the pathway differs, the energy levels of reactants and products stay the same, so overall enthalpy change remains unchanged.
This new mechanism may involve intermediates or surface-bound species that exist only in the catalysed route.
Efficiency depends on how reactants interact with the catalyst:
Solubility and phase behaviour of reactants
Required degree of molecular control (greater in homogeneous systems)
Desired ease of catalyst recovery (greater in heterogeneous systems)
Sensitivity to catalyst poisoning
Temperature and pressure conditions
Industries choose the system that offers the best balance of rate enhancement, selectivity, and operational practicality.
Practice Questions
Explain how a catalyst increases the rate of a chemical reaction.
(2 marks)
1 mark: States that a catalyst provides an alternative reaction pathway with lower activation energy.
1 mark: Explains that more particles have energy greater than the activation energy, increasing the frequency of successful collisions.
Figure 1 shows an enthalpy profile for a catalysed and an uncatalysed reaction.
Using your knowledge of catalysis, describe and explain:
the differences between the two curves
how a catalyst provides an alternative reaction pathway
how catalysis relates to homogeneous and heterogeneous systems.
(5 marks)
Award marks for the following valid points:
Identifies that the catalysed curve has a lower activation energy than the uncatalysed curve. (1 mark)
States that the overall enthalpy change remains the same for both pathways. (1 mark)
Explains that the catalyst provides an alternative pathway with lower activation energy. (1 mark)
Describes that in homogeneous catalysis the catalyst and reactants are in the same physical state, often forming intermediates. (1 mark)
Describes that in heterogeneous catalysis the catalyst is in a different physical state, and reactions occur on the catalyst surface. (1 mark)

