OCR Specification focus:
‘Explain the Boltzmann distribution and how temperature and catalysts affect the fraction of molecules exceeding activation energy, changing reaction rate.’
A collision-based view of chemical reactivity requires understanding how molecular energies are distributed in a sample, how few particles possess sufficient energy to react, and how altering conditions shifts this distribution.
The Boltzmann Distribution
The Boltzmann distribution describes how molecular energies are spread across the particles in a system at a given temperature. It shows that while most molecules have intermediate energies, only a small proportion possess sufficiently high energy to overcome the activation energy barrier for reaction.
Activation energy (Ea): The minimum energy that reacting particles must possess to form products via a successful collision.
A normal sentence must appear here to ensure clear separation between definition and further structured content.
Key Features of the Boltzmann Distribution
It is asymmetric, with a peak representing the most probable molecular energy.
It starts at the origin, as no molecules have zero energy.
It decreases gradually, showing a long tail of higher-energy molecules.
The area under the curve represents the total number of molecules.
The portion of molecules with energies greater than Ea determines the reaction rate.
Why Not All Collisions Lead to Reaction
Even when molecules collide, they react only if:
They possess energy ≥ activation energy.
They collide with correct orientation.
The Boltzmann distribution helps visualise the fraction of collisions that satisfy the energy requirement.
Temperature Effects on the Boltzmann Distribution
Increasing temperature does not simply increase all energies equally; instead, it reshapes the entire Boltzmann curve. This change has profound implications for reaction rate.
How Temperature Alters the Curve
When temperature rises:
The peak of the curve shifts rightwards to higher energies.
The peak becomes lower, as energies are more widely spread.
A significantly larger proportion of molecules exceeds Ea.
A normal sentence separates conceptual discussion from the next structured section.
Impact on Reaction Rate
Because more molecules have energies above Ea at higher temperatures:
The frequency of successful collisions increases.
Reaction rate rises sharply, which explains why even small temperature increases can produce major rate changes.
The curve’s tail becomes much more populated, meaning the fraction of effective collisions increases disproportionately relative to temperature change.
Visual Interpretation
On a Boltzmann diagram, the Ea threshold appears as a vertical line. When the temperature increases, the area under the curve to the right of this line becomes much larger, representing more energy-qualified molecules.
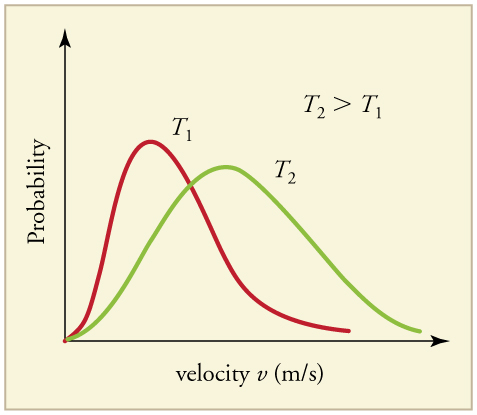
Maxwell–Boltzmann distributions at two temperatures. The higher-temperature curve is broader and shifted to higher energies, increasing the fraction of molecules with sufficient energy to react. The diagram is plotted against speed rather than energy, but illustrates the same principles relevant to activation energy. Source
Catalysts and the Boltzmann Distribution
A catalyst provides an alternative reaction pathway with a lower activation energy. It does not increase molecular energies or change the shape of the distribution.
Catalyst: A substance that increases reaction rate without being used up, by offering a lower-energy route for the reaction.
A normal sentence must appear here to maintain the required spacing before any further structured content.
How Catalysts Influence Ea
Lowering the activation energy means the vertical Ea line shifts leftwards on the energy axis of the Boltzmann distribution.
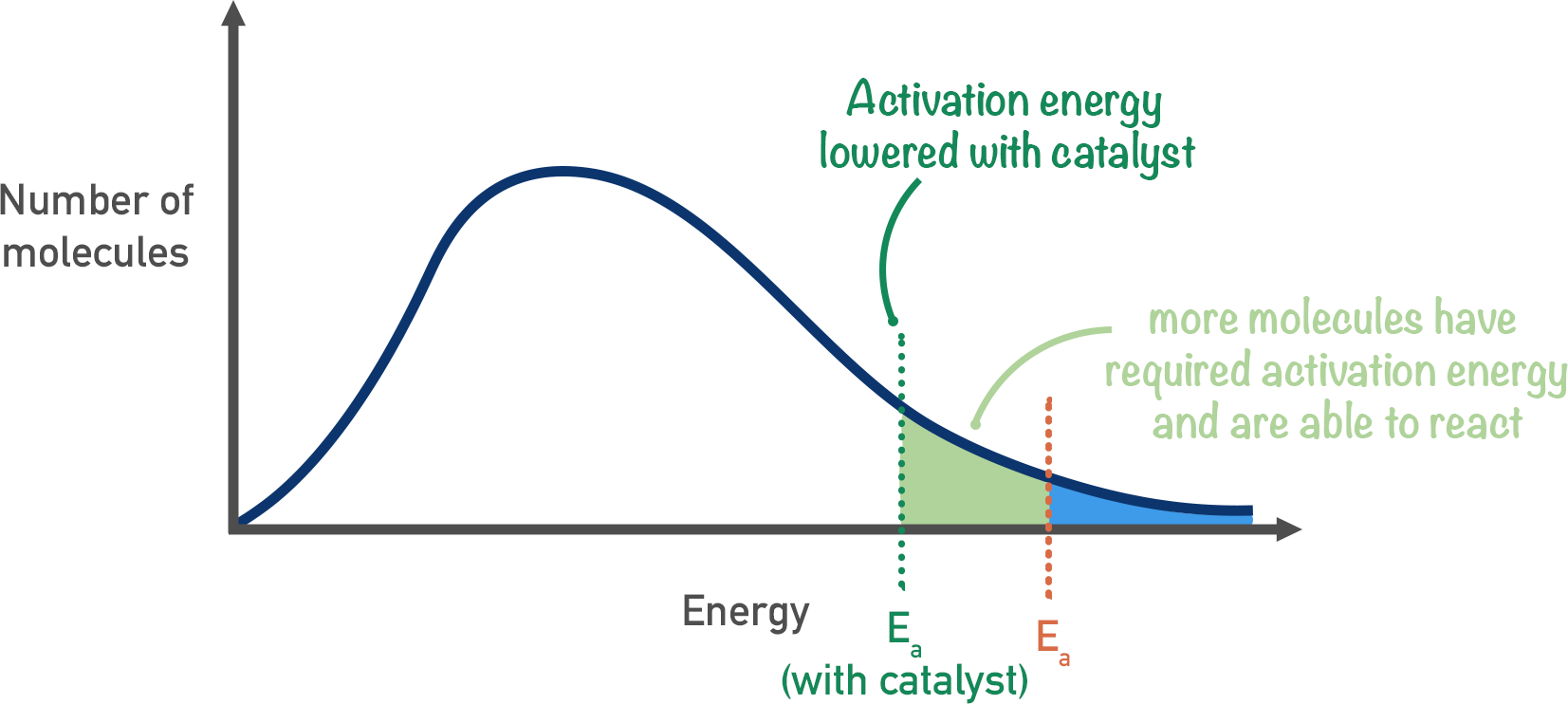
Maxwell–Boltzmann distribution showing the effect of a catalyst. Lowering the activation energy shifts the Ea line leftwards, increasing the proportion of molecules with enough energy to react. The curve itself remains unchanged because catalysts do not alter molecular energy distributions. Source
Visual Interpretation of Catalytic Effect
Catalysts do not alter the number, shape, or spread of molecular energies. Instead:
The energy requirement for reaction is reduced.
The proportion of molecules with sufficient energy becomes substantially larger.
This is why catalysts are valuable in industrial and environmental contexts: they allow reactions to proceed rapidly without the need for high temperatures.
Comparing Temperature and Catalytic Effects
Although both temperature increases and catalysts raise reaction rate, they do so in fundamentally different ways:
Increasing Temperature
Alters the entire distribution of molecular energies.
Increases the average kinetic energy.
Boosts both collision frequency and the fraction exceeding Ea.
Introducing a Catalyst
Leaves the distribution curve unchanged.
Reduces the energy barrier required for reaction.
Enables more molecules to react at the same temperature.
A normal sentence is included here to keep clarity before presenting the next concept.
Why the Boltzmann Distribution Is Central to Rate Theory
The Boltzmann distribution provides the essential link between particle energy and reaction feasibility. Because only a small fraction of molecules naturally have energies above Ea, understanding how to increase that fraction—through temperature or catalysts—is critical to predicting and controlling reaction rate.
Summary of Effects on Rate (Specification-Aligned)
Temperature increase: More molecules exceed activation energy, increasing reaction rate.
Catalyst addition: Provides a lower-energy pathway, raising the fraction of molecules able to react.
Both effects rely on the principles illustrated by the Boltzmann distribution, directly aligning with the specification requirement to explain how temperature and catalysts influence the fraction of molecules exceeding Ea.
FAQ
Reactions with low activation energies require only a small fraction of molecules to possess sufficient energy, so even at room temperature enough particles can react.
Reactions with high activation energies have only a very small proportion of molecules exceeding this threshold at lower temperatures, so heating is needed to shift more molecules into the high-energy region and enable reaction.
The distribution curve is highly sensitive near the activation energy threshold. Even a slight rise in temperature significantly increases the proportion of molecules in the high-energy tail.
This increase enhances the number of successful collisions far more than might be expected from the small change in average kinetic energy.
The concept applies most clearly to gases, where particle motion is relatively unrestricted and energy distribution follows predictable patterns.
In liquids, intermolecular forces introduce complexity, but the energy distribution still broadly follows Boltzmann behaviour.
In solids, limited particle mobility makes the model less directly applicable, though activation energy concepts remain relevant.
A catalyst lowers the activation energy, so the key question becomes how many molecules fall above this new threshold.
If the original distribution places most molecules close to but below the activation energy, even a modest reduction can dramatically increase the reacting fraction.
If molecules are mostly far below the threshold, the catalytic effect may be less pronounced.
The area represents the total number of molecules in the system, which remains constant unless matter is added or removed.
When temperature increases, the curve broadens and flattens, but it compensates so that the total area stays the same.
This preserves the relationship between molecular population and energy distribution while allowing shape changes that affect reaction rate.
Practice Questions
Explain why increasing the temperature of a reaction mixture increases the rate of reaction in terms of the Boltzmann distribution.
(2 marks)
1 mark: States that at higher temperature, a greater proportion of molecules have energy greater than or equal to the activation energy.
1 mark: States that this increases the frequency of successful collisions.
The diagram below shows a Maxwell–Boltzmann distribution curve for a reaction.
Describe how the curve and the fraction of molecules able to react would change if:
(a) the temperature is increased
(b) a catalyst is added
Explain your answers with reference to activation energy and molecular energy distribution.
(5 marks)
(a) Temperature increase:
1 mark: Peak moves to the right (higher energy).
1 mark: Peak becomes lower and broader.
1 mark: Greater proportion of molecules have energy greater than or equal to the activation energy.
(b) Addition of a catalyst:
1 mark: Catalyst lowers activation energy (Ea shifts left).
1 mark: Larger fraction of molecules now have sufficient energy to react, but the distribution curve itself does not change shape.

