OCR Specification focus:
‘Catalysts enable lower temperatures and reduced energy demand, improving sustainability, though some catalysts are toxic and require careful management.’
Catalysis has significant environmental and industrial importance because catalysts reduce energy consumption, support greener manufacturing, and lower environmental impact while still presenting challenges relating to toxicity, resource use and safe handling.
Catalysis and its Role in Sustainable Chemistry
Catalysis is a central concept in physical chemistry because it influences reaction rate, energy demand, and resource efficiency. A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Catalysts work by providing an alternative reaction pathway with a lower activation energy. This reduced energy requirement makes catalysis essential for sustainability in modern chemical industries.
Catalyst: A substance that increases reaction rate without being used up, providing an alternative pathway with lower activation energy.
The application of catalysis in sustainability focuses on reducing environmental impact, minimising waste, and lowering the carbon footprint of manufacturing processes. Catalytic processes operate in a range of systems, from small-scale laboratory reactions to large industrial plants.
A key benefit of catalysis is its influence on energy efficiency, allowing reactions to proceed at lower temperatures and sometimes lower pressures, decreasing fuel consumption and operational costs. Reduced energy demand directly links catalysis to sustainable practice, in accordance with the OCR specification for this subsubtopic.
On an enthalpy profile, a catalysed reaction has a lower activation energy than the uncatalysed reaction, so more particles have sufficient energy to react at a given temperature.
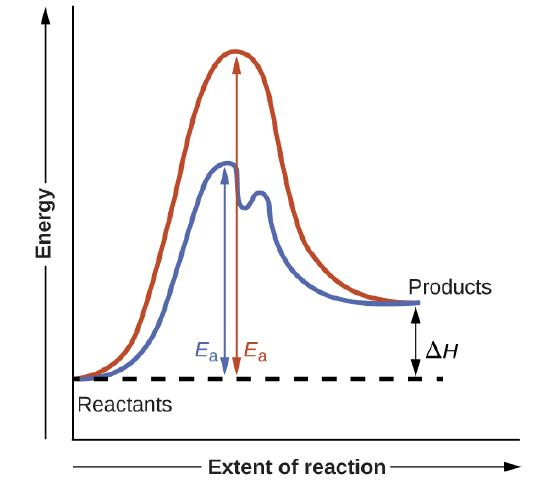
This diagram compares potential energy profiles for catalysed and uncatalysed reactions, highlighting the lower activation energy for the catalysed pathway. The inclusion of an intermediate step provides additional context beyond the OCR specification while reinforcing the alternative reaction mechanism. Source
How Catalysts Improve Sustainability
Catalysts improve sustainability through several interconnected mechanisms that reduce environmental burden and enhance industrial efficiency.
Lower Energy Requirements
Catalysts reduce the activation energy needed for a reaction, enabling it to occur at more moderate temperatures. Lower operating temperatures require less fuel, meaning:
Reduced carbon dioxide emissions from energy production
Lower operating costs
Less thermal stress on equipment, extending plant lifespan
Higher Reaction Efficiency
Catalysts help chemical reactions achieve higher atom economy, meaning a greater proportion of reactant atoms are incorporated into the final product.
Atom economy: The extent to which reactant atoms are converted into desired product; higher values indicate more efficient, less wasteful reactions.
A well-designed catalytic process generates fewer by-products, decreasing waste streams and reducing the environmental impact of disposal and purification.
Catalysts also enable continuous industrial processes, which are generally more efficient than batch processes and reduce downtime, further supporting sustainability goals.
Selectivity and Reduced By-products
Selective catalysts generate fewer unwanted products by directing reactions along a preferred pathway. Improved selectivity results in:
Lower purification demands
Less chemical waste
Lower water and solvent usage
More efficient use of raw materials
Use of Renewable Feedstocks
Some modern catalytic systems allow industries to utilise renewable feedstocks such as biomass-derived chemicals. Catalysts can:
Convert bioethanol into useful industrial chemicals
Aid the hydrogenation or oxidation of biomass-derived molecules
Support greener polymer production
By enabling the substitution of fossil-based raw materials, catalysts contribute to long-term sustainability.
Types of Catalysts and Sustainability Implications
Heterogeneous Catalysts
Heterogeneous catalysts exist in a different physical state from the reactants. They are commonly used in industry due to advantages such as:
Easy separation from products
Reusability
Lower contamination risk
Their surfaces provide active sites for reaction, improving efficiency. However, some heterogeneous catalysts include metals such as platinum, palladium, and nickel, whose extraction has environmental and ethical implications. Mining these metals can involve significant land disruption and energy consumption.
Homogeneous Catalysts
Homogeneous catalysts share the same physical state as reactants. Their high selectivity can reduce by-products, but their separation from reaction mixtures is more difficult. Sustainability considerations include:
Increased solvent use during separation
Higher risk of catalyst loss, generating waste
Potential toxicity, depending on catalyst species
Despite this, homogeneous catalysts are crucial in fine chemical synthesis, pharmaceuticals, and polymer chemistry due to their reaction specificity.
Biocatalysts and Enzymes
Biocatalysts, including enzymes, provide highly selective, mild-condition pathways for many reactions. Their sustainability benefits include:
Operation at low temperatures and neutral pH
Biodegradability
Reduced use of hazardous chemicals
Nevertheless, enzymes may require stabilising agents or specific conditions to maintain activity, which can complicate industrial use.
Environmental and Sustainability Challenges of Catalysis
Although catalysts improve energy efficiency and reduce waste, the OCR specification emphasises that some catalysts are toxic and require careful management. Sustainability is therefore not solely about benefits; limitations must also be considered.
Toxicity and Environmental Risk
Some metal catalysts (e.g., cobalt, nickel, chromium) pose risks if released into the environment. These dangers may include:
Bioaccumulation in ecosystems
Harm to aquatic organisms
Health risks to humans during handling or disposal
Stringent waste management protocols and catalyst recovery systems are often necessary.
Scarcity of Catalyst Materials
Many industrial catalysts use rare-earth metals or precious metals, which are limited resources. Sustainability issues include:
High extraction energy costs
Geopolitical supply concerns
Long-term resource depletion
Recycling catalysts through regeneration and recovery processes is a major focus in green chemistry.
Disposal and Regeneration
Catalyst regeneration extends the lifespan of catalytic systems but requires additional processing. Disposal must be carefully managed to avoid environmental contamination.
Spent catalysts may contain toxic residues
Regeneration processes consume energy
Inefficient regeneration can reduce catalyst performance
Car exhaust catalytic converters use platinum, palladium or rhodium catalysts to convert carbon monoxide, nitrogen oxides and unburnt hydrocarbons into less harmful gases, reducing air pollution and improving sustainability.
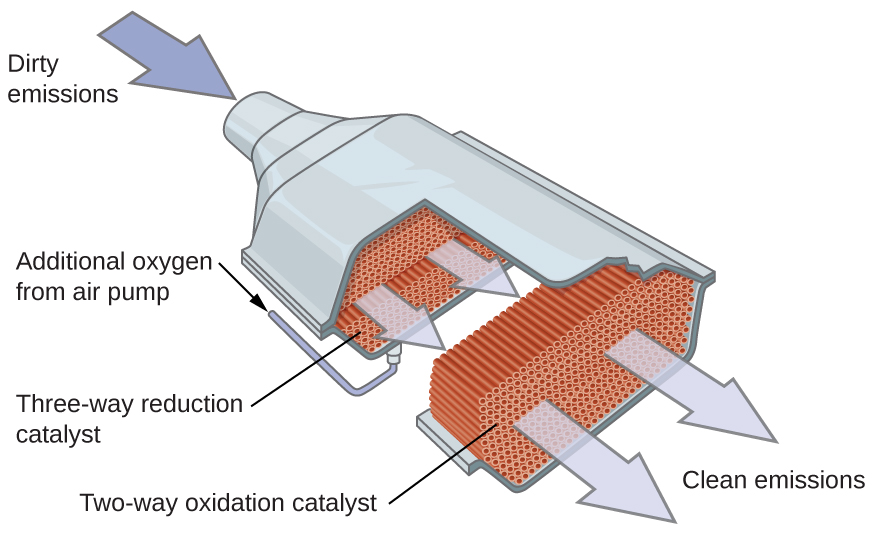
This diagram shows the structure and function of a three-way catalytic converter, illustrating how exhaust gases pass over platinum-group metal catalysts to reduce pollutant emissions. Some reaction details included exceed OCR requirements but support understanding of catalysis in sustainability contexts. Source
Catalysis and Sustainable Industrial Practice
To address sustainability challenges, modern industries employ strategies such as:
Designing catalysts from abundant, non-toxic metals
Developing solid acid catalysts to reduce hazardous liquid acids
Recovering and recycling precious metal catalysts
Implementing alternative catalytic pathways using enzymes
Catalysis therefore represents a balance between technological benefits and responsible management of materials, aligning closely with the OCR specification emphasis on energy reduction and safe handling.
FAQ
Catalysts enable more efficient reaction pathways that minimise the formation of toxic or energy-intensive by-products. This reduces the need for downstream purification processes, which are often major contributors to energy use.
Many catalytic processes also allow plants to shift from batch to continuous operation, improving heat management, reducing waste, and lowering operational downtime.
Platinum-group metals offer exceptionally high surface activity and stability under harsh industrial conditions, making them difficult to replace.
Their ability to remain effective for long periods offsets some sustainability concerns, although recycling systems are essential to recover metals and reduce environmental impact from mining.
Chemists are developing catalyst supports that maximise surface area, meaning less of the rare metal is needed.
Alternative strategies include:
Designing catalysts from abundant transition metals.
Using nanostructuring to improve activity.
Employing biocatalysts when suitable for mild-condition reactions.
Deactivation may occur through poisoning, fouling, or structural changes. Plants monitor reaction rates, selectivity and product compositions to identify declines in catalytic performance.
Management approaches include periodic regeneration, mechanical cleaning, or replacing specific catalyst modules while maintaining continuous operation.
Catalyst recycling reduces the need to extract new metals, lowering environmental disruption and energy consumption associated with mining.
Recycling also ensures valuable or scarce materials remain in circulation, helping stabilise supply chains while preventing hazardous catalyst waste from entering the environment.
Practice Questions
Explain how the use of a catalyst can improve the sustainability of an industrial chemical process.
(3 marks)
Award 1 mark for each valid, distinct point (maximum 3).
Catalyst lowers activation energy, allowing the reaction to proceed at lower temperature.
Lower temperature reduces energy demand and fuel consumption.
Decreased energy use reduces carbon emissions / environmental impact.
Catalysts are widely used to improve the efficiency and sustainability of industrial reactions, but they also present several challenges.
Discuss the benefits and limitations of catalysts in relation to sustainability, including at least one benefit and one limitation.
(6 marks)
Award marks for the following points, up to a total of 6.
Benefits (maximum 4 marks):
Lower activation energy decreases required operating temperature.
Reduced energy consumption improves sustainability and lowers carbon emissions.
Catalysts increase reaction rate, improving process efficiency.
Higher selectivity reduces waste and unwanted by-products.
Enables use of renewable feedstocks in some catalytic processes.
Limitations (maximum 3 marks):
Some catalysts are toxic and require careful handling and disposal.
Precious metal catalysts (e.g., platinum, palladium) are scarce and environmentally damaging to extract.
Regeneration and disposal of catalysts may require additional energy or produce hazardous waste.
Quality of discussion (1 mark):
Award 1 additional mark for coherent comparison of benefits and limitations demonstrating clear understanding of sustainability implications.

