OCR Specification focus:
‘A catalyst increases the rates of forward and reverse reactions equally, leaving the equilibrium position unchanged while reaching equilibrium faster.’
Introduction
Catalysts influence how quickly equilibrium is reached by accelerating both forward and reverse reactions equally, yet they do not alter the equilibrium position or chemical composition at equilibrium.
Catalysts in the Context of Dynamic Equilibrium
In a dynamic equilibrium, the forward and reverse reactions occur at equal rates, and the concentrations of reactants and products remain constant over time. When a catalyst is introduced into such a system, it interacts with the reaction pathway rather than the equilibrium composition.
A catalyst is a substance that increases the rate of a chemical reaction without being consumed. Its central role in equilibrium systems is to provide an alternative route with lower activation energy, allowing more molecules to react per unit time.
How Catalysts Affect Reaction Rates
Catalysts enhance reaction rates by altering the mechanism. This change affects both directions of a reversible reaction equally.
Activation energy: The minimum energy required for particles to react.
Because activation energy is lowered in both directions, the system can reach equilibrium more quickly but without altering the actual equilibrium composition.
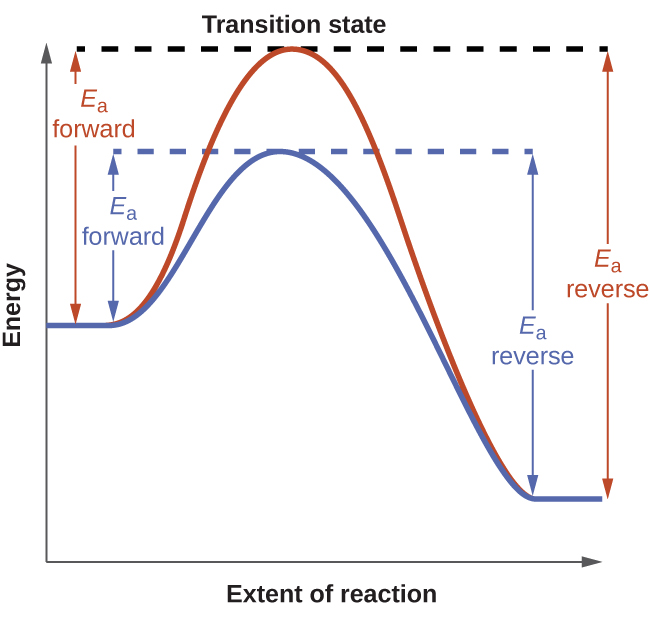
The diagram compares uncatalysed and catalysed pathways, showing how a catalyst lowers activation energy in both directions while leaving the enthalpy change and equilibrium position unchanged. Source
After introducing a definition block, it is essential to explore the mechanistic consequences for equilibrium. The rate increase applies to both forward and reverse reactions because each step in a reversible mechanism is influenced by the catalyst.
Catalytic Action in Forward and Reverse Reactions
A catalyst facilitates the formation of an intermediate or transition complex, reducing the energy barrier in both reaction directions. As a result:
Forward reaction rate increases because more reactant molecules possess sufficient energy to form products.
Reverse reaction rate increases because product molecules also more readily convert back into reactants.
Overall equilibrium is reached faster, with no shift in equilibrium composition.
This equal effect on rates is crucial. If a catalyst affected only one direction, it would alter the ratio of reactants to products, contradicting the principle that catalysts do not change equilibrium constants.
Why the Equilibrium Position Does Not Change
The equilibrium position refers to the relative proportions of reactants and products at equilibrium. This position is governed solely by thermodynamic factors such as enthalpy and entropy. Catalysts do not modify these properties.
Equilibrium constant (Kc): A ratio of product to reactant concentrations at equilibrium, each raised to the power of their stoichiometric coefficients.
A sentence placed here ensures spacing between definition blocks before introducing additional structured content.
Energy Profile Representation of Catalytic Effects
Energy profile diagrams visually illustrate how catalysts influence reaction pathways. In these diagrams:
The reactants and products maintain identical energy levels, meaning ΔH is unchanged.
The activation energy peak is lowered for both directions.
The profile displays two pathways: uncatalysed (higher peak) and catalysed (lower peak).
This lower activation energy enables a greater proportion of molecules to successfully collide and react per second.
Homogeneous and Heterogeneous Catalysts in Equilibrium Systems
The OCR specification requires understanding of homogeneous and heterogeneous catalysts in relation to reversible reactions:
Homogeneous Catalysts
These catalysts are in the same phase as the reactants, often forming intermediates. They provide alternative pathways and can be particularly effective in solution-phase reactions.
Heterogeneous Catalysts
These catalysts exist in a different phase, typically solid catalysts interacting with gaseous or aqueous reactants. Their mechanism frequently involves adsorption, reaction on the surface, and desorption.
Although the catalytic mechanisms differ, the outcome for equilibrium systems remains the same: both directions of the reaction are accelerated equally.
Key Features of Catalysts and Equilibrium Position
The essential conceptual points for this subsubtopic can be summarised as follows:
Catalysts speed up both forward and reverse reactions equally.
They do not alter ΔH, so thermodynamic properties remain unchanged.
The equilibrium constant remains the same, ensuring no shift in the equilibrium position.
The system reaches equilibrium faster, which is especially useful in industrial processes.
Catalysts remain chemically unchanged at the end of the reaction.
Industrial Importance of Catalysts in Equilibrium Contexts
Although catalysts do not change equilibrium position, they have considerable industrial value. Faster attainment of equilibrium allows:
Quicker production cycles.
Lower operating temperatures in some cases, reducing energy consumption.
Improved economic efficiency without sacrificing equilibrium yield.
Common industrial catalysts include iron in the Haber process and vanadium(V) oxide in the contact process, each providing a lower-energy reaction route while leaving the equilibrium position unchanged.
Practical Considerations in Catalytic Equilibrium Systems
When applying catalysts in reversible reactions, several factors must be considered:
Catalyst poisoning, where impurities block active sites and reduce efficiency.
Surface area availability for heterogeneous catalysts, controlling reaction accessibility.
Reaction conditions, such as temperature and pressure, which influence catalyst performance but not its effect on equilibrium position.
These considerations ensure that catalysts function effectively, enabling faster establishment of equilibrium without modifying the chemical balance dictated by thermodynamics.
At the same temperature and pressure, a catalysed equilibrium system therefore reaches constant concentrations in a shorter time, but those final concentrations are identical to the uncatalysed case.
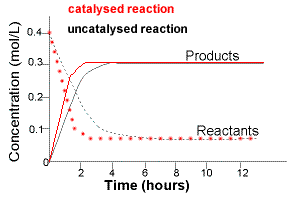
The graph illustrates that catalysed and uncatalysed systems reach the same equilibrium concentrations, but the catalysed reaction reaches equilibrium faster due to increased forward and reverse rates. Source
FAQ
A catalyst changes the mechanism by introducing one or more alternative steps with lower activation energies. These steps may involve different intermediates or transition states than the uncatalysed pathway.
Because both forward and reverse mechanisms are altered proportionally, the catalyst changes the route taken but not the thermodynamic properties governing equilibrium.
A catalyst affects the reaction pathway, not the kinetic energy of the particles themselves. The Boltzmann distribution, which describes particle energies at a given temperature, remains unchanged.
As a result, the fraction of particles able to react increases, but no thermodynamic quantities linked to equilibrium are modified.
Both types of catalysts accelerate equilibrium attainment, but their modes of action differ.
Homogeneous catalysts form intermediates within the same phase as the reactants.
Heterogeneous catalysts provide surface sites for adsorption, reaction, and desorption.
Despite these differences, neither type affects the final equilibrium composition.
Yes, catalysts may sometimes enable minor side reactions by lowering activation energies for alternative pathways.
However, for the principal reversible reaction, the catalyst still lowers activation energies for both forward and reverse directions equally, preserving the main equilibrium position.
Catalyst poisoning reduces the number of available active sites, slowing both forward and reverse reactions.
This does not change the equilibrium position but increases the time needed to reach it, reducing process efficiency and potentially increasing energy consumption to compensate.
Practice Questions
A catalyst is added to a reversible reaction mixture at equilibrium.
State and explain the effect, if any, that the catalyst has on:
a) the equilibrium position
b) the time taken to reach equilibrium
(2 marks)
a)
No change to the equilibrium position. (1 mark)
b)
Equilibrium is reached faster because the catalyst increases the rate of both forward and reverse reactions equally. (1 mark)
The reaction between substances X and Y is reversible. A catalyst is introduced, and the reaction mixture reaches equilibrium more quickly.
Using an energy profile diagram and kinetic reasoning, explain:
a) why the catalyst speeds up both the forward and reverse reactions
b) why the equilibrium constant remains unchanged
c) how the activation energies for the catalysed and uncatalysed pathways compare
(5 marks)
a)
A catalyst provides an alternative pathway with lower activation energy. (1 mark)
Lower activation energy increases the rate of both forward and reverse reactions. (1 mark)
b)
The equilibrium constant depends only on temperature and the enthalpy change of the reaction, neither of which is affected by the catalyst. (1 mark)
c)
The catalysed pathway has a lower activation energy than the uncatalysed pathway for both directions. (1 mark)
Energy levels of reactants and products remain unchanged, so the enthalpy change is unaffected. (1 mark)

