OCR Specification focus:
‘Write Kc expressions for homogeneous reactions and calculate Kc from equilibrium concentrations; use magnitude of Kc to estimate equilibrium position.’
A chemical equilibrium’s position can be quantified using the equilibrium constant Kc, allowing chemists to assess how far a reaction proceeds and how concentrations relate at equilibrium.
Understanding the Equilibrium Constant Kc
The equilibrium constant Kc is a numerical value that describes the ratio of concentrations of products to reactants at equilibrium for a reversible reaction. It stems directly from the balanced chemical equation and applies specifically to homogeneous equilibria, where all species are in the same physical state. Kc is central to analysing equilibrium systems because it enables prediction of equilibrium position and comparison of different reactions under consistent conditions.
Equilibrium Constant Kc: The ratio of equilibrium concentrations of products to reactants, each raised to the power of their stoichiometric coefficients, for a homogeneous reaction.
Kc provides a quantitative description that complements qualitative predictions made using Le Chatelier’s principle.
Writing Kc Expressions
For any homogeneous reversible reaction of the form
aA + bB ⇌ cC + dD, the equilibrium constant expression is constructed systematically.
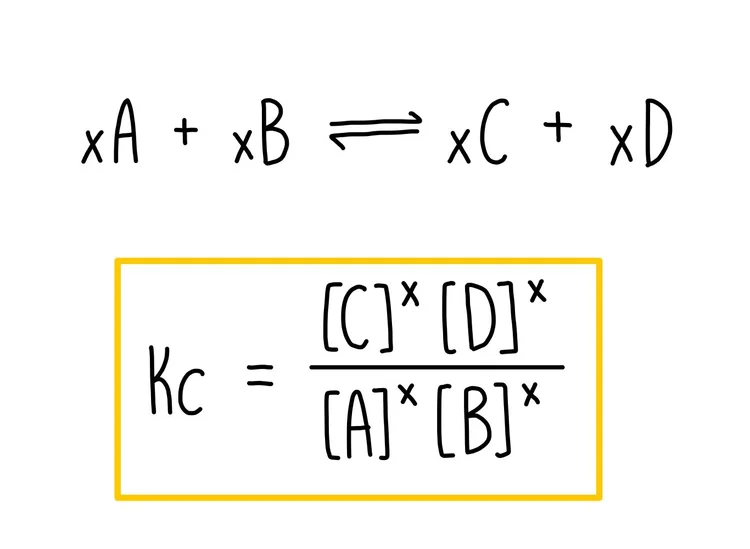
This diagram shows a general homogeneous equilibrium and the corresponding Kc expression, with products in the numerator and reactants in the denominator. The exponents on each concentration match the stoichiometric coefficients in the balanced equation. The image contains no extra detail beyond what is required to understand how to write Kc for OCR A-Level Chemistry. Source
Equilibrium Constant (Kc) = ([C]ᶜ [D]ᵈ) / ([A]ᵃ [B]ᵇ)
[C] = Equilibrium concentration of product C in mol dm⁻³
[D] = Equilibrium concentration of product D in mol dm⁻³
[A] = Equilibrium concentration of reactant A in mol dm⁻³
[B] = Equilibrium concentration of reactant B in mol dm⁻³
Stoichiometric coefficients determine the powers applied to each concentration term, making accurate balanced equations essential before constructing any Kc expression.
A Kc expression is only written for species in the same physical state. OCR focuses on homogeneous systems, meaning all reactants and products typically appear in the expression. Heterogeneous reactions, such as those involving solids, are not included in Kc at this level.
Key Features of Kc Expressions
Products appear in the numerator, reactants in the denominator.
Concentrations at equilibrium only, never initial concentrations.
Units vary depending on the reaction stoichiometry; some Kc values are dimensionless.
Temperature-dependent, meaning each reaction has a specific Kc at a specific temperature.
Determining Kc from Equilibrium Concentrations
Once the equilibrium concentrations of all relevant species are known, Kc can be calculated directly using the constructed expression. The practical challenge lies in obtaining accurate concentration data, but the mathematical substitution itself is straightforward.
Typical sources of equilibrium concentration data include:
analytical measurements such as titration of samples withdrawn at equilibrium
spectroscopy for coloured species
pH measurements for acid–base systems
initial concentration and stoichiometric change data derived from reaction tables
After substituting values into the Kc expression, the calculation produces a numerical value associated with a specific temperature. Students must remember that only equilibrium concentrations are valid inputs.
Using the Magnitude of Kc to Estimate Equilibrium Position
The OCR specification emphasises interpreting what Kc values imply about the position of equilibrium.
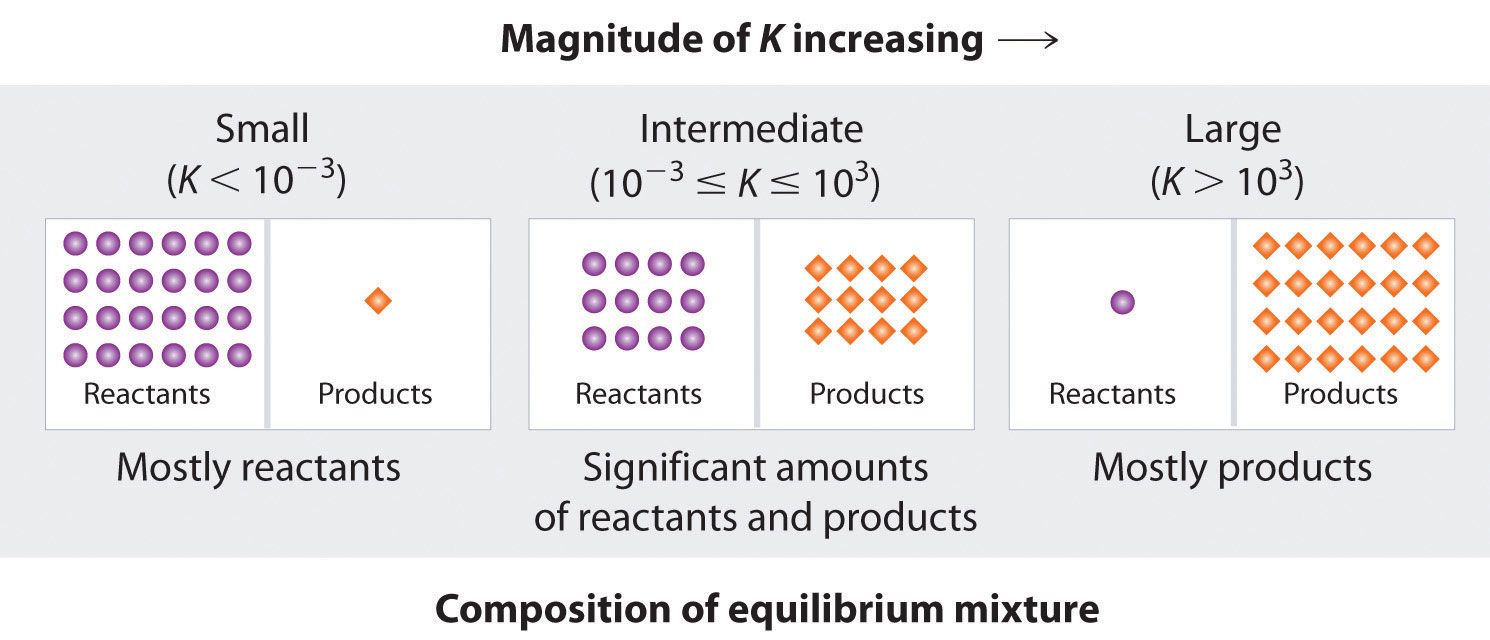
This diagram shows how the composition of an equilibrium mixture changes as K increases from very small to very large values. When K is small, the boxes contain mostly reactant particles; when K is large, they contain mostly product particles, with an intermediate case in between. The numerical K ranges shown add extra quantitative detail but remain consistent with the qualitative descriptions required by the OCR specification. Source
Interpreting the Size of Kc
Kc >> 1
The equilibrium mixture contains mostly products.
The forward reaction is strongly favoured.
Kc << 1
The equilibrium mixture contains mostly reactants.
The reverse reaction is strongly favoured.
Kc ≈ 1
Significant amounts of both reactants and products remain.
Neither direction is strongly favoured.
Kc therefore acts as a quantitative descriptor of equilibrium composition and can be used to compare the behaviour of different reactions under similar temperature conditions.
Factors Affecting Kc
Although changes in concentration or pressure affect the position of equilibrium, they do not change the equilibrium constant itself. This point is essential for explaining why Kc remains constant provided the temperature does not change.
However, temperature has a direct effect on Kc:
For endothermic forward reactions, increasing temperature increases Kc.
For exothermic forward reactions, increasing temperature decreases Kc.
Understanding these relationships is vital when predicting equilibrium shifts and explaining industrial compromise conditions.
Practical Considerations When Using Kc
To use Kc effectively in chemical analysis, students must be comfortable with:
identifying homogeneous systems
constructing correct Kc expressions
working with equilibrium concentration data
interpreting numerical values in context
Bullet-point reminders for accuracy:
Always write the balanced equation first.
Check that all species are in the same state.
Substitute equilibrium concentrations only.
Interpret the result in terms of relative amounts of reactants and products.
Kc is one of the most important tools in equilibrium chemistry, providing a direct quantitative link between chemical equations and measurable composition of equilibrium mixtures.
FAQ
Changing the coefficients directly changes the powers used in the Kc expression. Doubling all coefficients squares every concentration term, altering both the value and units of Kc.
This is why Kc values are only comparable when based on the same balanced chemical equation.
OCR focuses on homogeneous equilibria to simplify analysis, as all species share the same state and concentration can be clearly defined.
In heterogeneous systems, solids and pure liquids have constant densities and are therefore omitted from expressions. Their inclusion would not affect Kc and adds unnecessary complexity for this level.
Changes in concentration or pressure shift equilibrium position without altering the ratio of products to reactants at the new equilibrium.
Temperature, however, changes the relative energetics of the forward and reverse reactions, meaning a different ratio of products to reactants is established, creating a new Kc.
Units result from combining concentration terms according to the stoichiometric powers in the Kc expression.
If the total powers of products and reactants cancel, the final units simplify to none.
This depends entirely on the reaction equation, not on the equilibrium mixture itself.
Common difficulties include:
Inaccurate equilibrium concentration measurements
Temperature fluctuations altering Kc during sampling
Side reactions or incomplete equilibrium establishment
Reliable Kc values require stable temperature control, consistent analytical techniques, and confirming that equilibrium has been reached before sampling.
Practice Questions
A reversible homogeneous reaction is shown below:
A + 2B ⇌ C
Write the expression for the equilibrium constant Kc for this reaction.
(2 marks)
Award one mark for each of the following:
Correct numerator: [C]
Correct denominator: [A][B]²
Full expression required for 2 marks:
Kc = [C] / ([A][B]²)
The following equilibrium is established at a fixed temperature:
2X ⇌ Y + Z
A student investigates how the magnitude of Kc relates to the position of equilibrium.
Explain how the equilibrium composition changes when:
a) Kc is much greater than 1
b) Kc is much less than 1
c) The temperature is increased for an endothermic forward reaction
Your answer should refer to relative amounts of reactants and products.
(5 marks)
a) When Kc >> 1
States that equilibrium mixture contains mostly products (1 mark)
Forward reaction favoured (1 mark)
b) When Kc << 1
States that equilibrium mixture contains mostly reactants (1 mark)
Reverse reaction favoured (1 mark)
c) Increasing temperature for endothermic forward reaction
States that increasing temperature increases Kc because the forward reaction is endothermic (1 mark)
(Any 5 correct points = 5 marks)

