OCR Specification focus:
‘A dynamic equilibrium exists in a closed system when forward and reverse reaction rates are equal and concentrations remain constant.’
Dynamic equilibrium is a fundamental concept describing how reversible reactions behave when no substances can enter or leave the system. This topic explains how equal reaction rates maintain constant composition.
What Is Meant by Dynamic Equilibrium?
A dynamic equilibrium is reached in a reversible reaction when the forward and reverse reaction rates become equal, so the measurable concentrations of reactants and products remain constant over time. Although the composition appears unchanged, the system remains highly active at the molecular level.
Dynamic equilibrium: A state in a closed system in which the forward and reverse reaction rates are equal, resulting in constant concentrations of reactants and products.
Before equilibrium can be established, the system must be closed to prevent loss or gain of matter.
Closed system: A system in which no substances can enter or leave, but energy transfer is still possible.
These conditions ensure that any observed changes in composition result solely from reaction processes rather than material exchange with the surroundings.
Requirements for Establishing Dynamic Equilibrium
For a reversible reaction to reach equilibrium, three key conditions must be met:
Closed system so mass remains constant
Reversibility of reaction, allowing both forward and backward processes
Sufficient time for the rates to adjust until equality is reached
A reversible reaction is indicated by the symbol ⇌, showing that reactants form products and products simultaneously reform reactants.
After these requirements are fulfilled, the system progresses towards a state where macroscopic observables remain unchanged.
Molecular Interpretation of Equilibrium
Although no net change in concentration is recorded at equilibrium, the system remains in constant flux. Particles continue to collide, react and interconvert.
Equal Rates at Equilibrium
At equilibrium:
The rate of the forward reaction equals the rate of the reverse reaction.
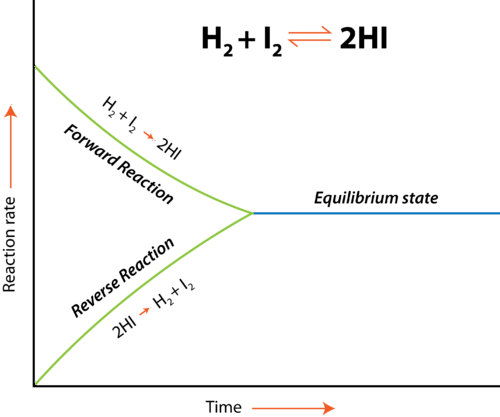
A graph showing how forward and reverse reaction rates change until they become equal at equilibrium, illustrating the dynamic balance within a closed system. Source
The concentrations stay constant, not because reactions stop, but because their effects cancel out.
Rate of reaction: The change in concentration of a reactant or product per unit time.
A single sentence separating definition blocks: Equilibrium is therefore a dynamic balance, not a static halt in chemical activity.
Features of a Dynamic Equilibrium
Key characteristics of a dynamic equilibrium include:
Constant concentrations of all species in the reaction mixture
Ongoing microscopic change despite macroscopic constancy
Forward and reverse processes continuing indefinitely
Dependence on external conditions such as temperature and pressure, which determine the equilibrium position (but do not alter the fact that rates are equal at equilibrium)
Students should differentiate clearly between constant and equal: concentrations are constant, but rates are equal.
Graphical and Conceptual Representation
Dynamic equilibrium can be visualised using concentration–time or rate–time graphs:
Concentration–time graphs
Initially, concentration of reactants decreases while products increase.
Eventually, both stabilise at constant values once equilibrium is reached.
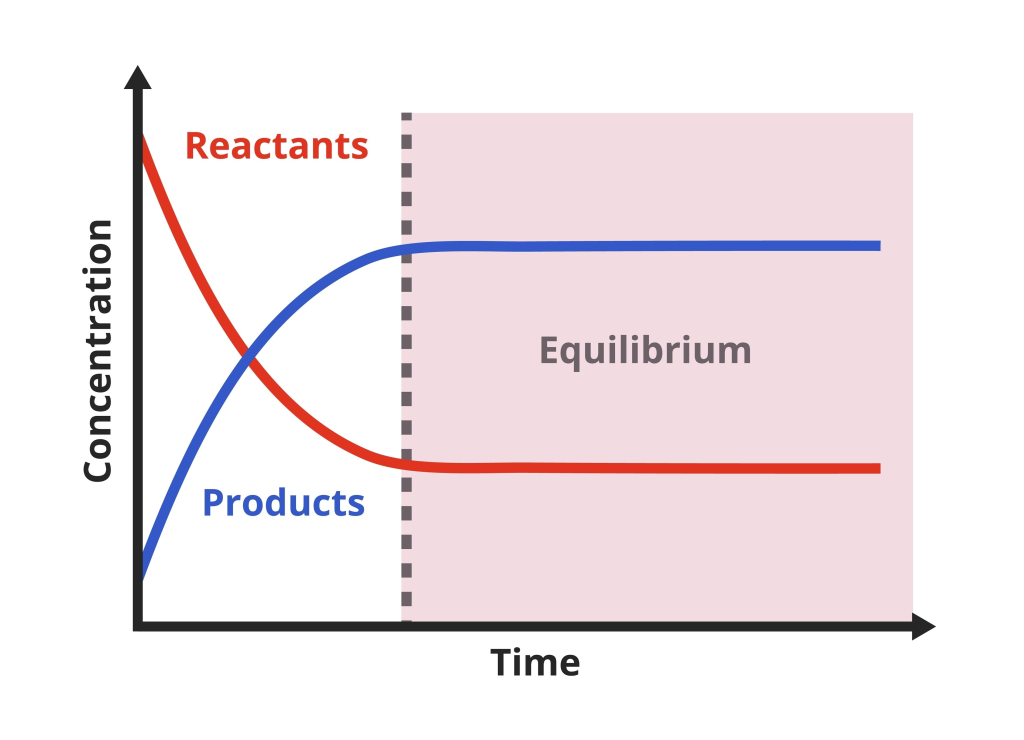
A concentration–time graph showing reactant and product concentrations levelling off once dynamic equilibrium is reached in a closed system. Source
Rate–time graphs
The forward reaction rate decreases as reactants are consumed.
The reverse reaction rate increases as products accumulate.
When the lines meet, their equality marks attainment of equilibrium.
These graphical perspectives help link macroscopic observations to molecular behaviour.
How Dynamic Equilibrium Responds to Conditions
Although this topic does not yet address Le Chatelier’s principle, it is important to recognise that equilibrium requires constant conditions. If factors such as temperature or pressure change, the equilibrium position shifts to a new set of constant concentrations, but the system will again settle where forward and reverse rates eventually match.
Importance of a Closed System
A closed system is crucial because:
Loss of gaseous reactants or products prevents equilibrium from being reached.
Addition of new substances disrupts concentration balance.
Temperature changes are permissible, but they alter equilibrium conditions rather than preventing equilibrium.
Reversible reaction: A reaction in which products can reform reactants under the same conditions.
A single sentence separating definition blocks: In a closed system, reversibility ensures the reaction can move in both directions until equilibrium is achieved.
Why Equilibrium Concentrations Remain Constant
The constancy of concentration at equilibrium arises because:
The rate of formation of products equals the rate of their conversion back into reactants.
Any disturbance within the system is corrected by the reaction adjusting in both directions until balance is restored.
No net change occurs despite substantial molecular motion.
This balance allows chemists to predict reaction behaviour, control industrial processes and interpret experimental data.
Distinguishing Dynamic Equilibrium from Completion
Students should understand the contrast between:
Reactions that go to completion: Reactants are used up; no reverse reaction of significance occurs.
Reactions establishing equilibrium: Both reactants and products are present at all times; the reverse reaction is substantial.
Dynamic equilibria are characteristic of many important processes, including acid–base reactions, phase equilibria and gas–phase reversible reactions.
Key Takeaways for OCR Study
A dynamic equilibrium occurs in a closed system when forward and reverse reaction rates are equal.
Concentrations remain constant, not because reactions stop, but because their rates balance.
Reversibility and closed-system conditions are essential requirements.
Molecular-level activity continues throughout, defining the system as dynamic rather than static.
FAQ
Dynamic equilibrium depends on frequent, successful collisions between particles in both the forward and reverse directions. As product concentration builds, more reverse collisions lead to product-to-reactant conversion.
The system reaches equilibrium once collision frequencies produce equal reaction rates in both directions, maintaining constant composition while molecular activity continues.
A reaction may not reach equilibrium if gases escape, products precipitate out, or a side reaction removes species from the mixture.
Dynamic equilibrium requires all reacting particles to remain available, meaning any physical or chemical process that removes material prevents the system from balancing its rates.
Higher temperatures provide particles with more kinetic energy, increasing collision frequency and helping the system reach equal rates more quickly.
Lower temperatures slow particle movement, reducing reaction rates and increasing the time required for equilibrium to become established.
A reaction is likely at equilibrium when measurable properties stop changing. These may include:
constant colour intensity
constant pressure in a sealed gaseous system
constant pH for acid–base equilibria
These observations show the macroscopic state is steady, even though molecular changes continue.
A closed system ensures mass remains constant, allowing concentration changes to arise solely from reaction events. This makes measurements reproducible and meaningful.
If material enters or escapes, rate balance cannot be reliably monitored, and any analysis of equilibrium concentrations or graphical behaviour becomes invalid.
Practice Questions
State what is meant by a dynamic equilibrium and explain why it can only occur in a closed system.
(2 marks)
1 mark: Dynamic equilibrium is when the forward and reverse reaction rates are equal.
1 mark: It can only occur in a closed system because no substances can enter or leave.
A reversible reaction reaches dynamic equilibrium in a closed container.
Describe, in terms of rates and concentrations, what happens inside the system once equilibrium has been established.
Explain why the equilibrium is described as dynamic, and comment on the macroscopic observations a chemist would make during this process.
(5 marks)
Award marks for the following points (any valid wording acceptable):
1 mark: At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction.
1 mark: Concentrations of reactants and products remain constant.
1 mark: The equilibrium is dynamic because both reactions continue to occur.
1 mark: There is continuous molecular activity or interconversion of reactants and products.
1 mark: No observable macroscopic change is seen; the system appears unchanged to the chemist.

