OCR Specification focus:
‘Industrial conditions balance equilibrium yield with reaction rate, safety and economic factors, exemplified by processes such as the Haber process.’
Industrial chemistry requires balancing equilibrium yield and reaction rate with economic and safety constraints. Conditions cannot simply maximise yield; they must also ensure feasible costs and acceptable reaction times.
Industrial Compromises in Equilibrium and Rate
Industrial reactions rarely run under conditions that give the highest possible yield predicted by equilibrium alone. Instead, chemical engineers choose conditions offering a practical compromise between equilibrium position, rate of reaction, cost, and plant safety. This reflects the specification requirement that industrial conditions must balance equilibrium yield with reaction rate, safety, and economics, as illustrated in processes such as the Haber process.
Equilibrium Considerations in Industry
Chemical equilibria involve reversible reactions where the position of equilibrium determines the proportions of reactants and products present at equilibrium. Industrially, manipulating conditions using Le Chatelier’s principle influences equilibrium yield. However, applying extreme conditions to maximise product formation is often impractical.
Key equilibrium-related influences include:
Temperature: Affects both equilibrium position and the rate of reaction.
Pressure: Particularly significant for reactions involving gases with differing numbers of moles.
Concentration: Adjusting feed composition can shift equilibrium to favour product formation.
Catalysts: Improve rate without altering equilibrium position.
Rate Considerations in Industrial Processes
A reaction with a favourable equilibrium position may still be commercially useless if the rate of reaction is too slow at chosen conditions. For industrial processes, rate must be high enough to produce product at an economically viable scale.
Rate is influenced by:
Temperature
Pressure
Catalyst efficiency and lifespan
Surface area (for heterogeneous catalysts)
The Interplay Between Equilibrium and Rate
Many industrial reactions involve exothermic forward reactions, meaning lower temperatures favour higher yield according to Le Chatelier’s principle.
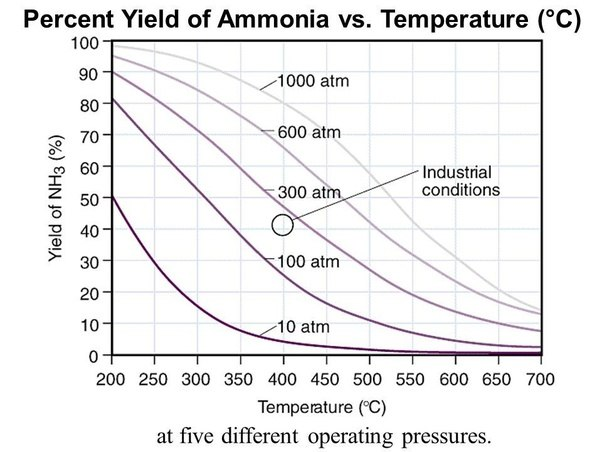
Graph showing the equilibrium percentage yield of ammonia against temperature for several pressures, illustrating that lower temperatures increase yield while higher pressures raise yield at any temperature. Includes numerical detail beyond the specification but remains consistent with the industrial compromise discussed. Source
However, low temperatures lead to reduced reaction rates. Conversely, higher temperatures increase rate but reduce equilibrium yield.
Industrial chemists therefore select a temperature that:
Is high enough to achieve an acceptable rate
Is low enough to avoid excessively lowering yield
Does not compromise catalyst stability
Remains economically and safely manageable
A similar compromise occurs with pressure. Very high pressures may significantly increase yield for reactions producing fewer moles of gas, but they also dramatically raise equipment and energy costs while increasing safety risks.
Case Study Context: The Haber Process (Specification Example)
The syllabus highlights the Haber process, where nitrogen reacts with hydrogen to form ammonia in a reversible, exothermic reaction.
####################################### Image: insert image from https://byjus.com/chemistry/haber-process/
Identification: A coloured horizontal flow diagram showing nitrogen and hydrogen feeds, a compressor, a reactor labelled with 400–450 °C and 150–200 atm, and a recycle loop, positioned just below the sentence “Let us take a look at the diagram below.”
Caption: Diagram of the Haber process showing gas feeds, compression, catalytic reaction, condensation of ammonia, and recycling of unreacted gases. This illustrates how industry improves yield without extreme conditions. Some additional industrial detail is present but does not affect core specification content. #######################################
Although equilibrium yield increases at low temperature and high pressure, industry uses moderate temperatures and lower-than-maximum pressures because:
Very low temperatures give unacceptably slow rates.
Extreme pressures require expensive reinforced equipment.
Catalyst performance depends on operating within a stable temperature range.
This industrial example reflects the essential balance between equilibrium yield and reaction rate described by the specification.
Key Factors Guiding Industrial Compromise
Industrial plant operation must consider the combined effect of several technical, economic, and safety factors. Common considerations include:
1. Economic Considerations
Energy costs: High temperatures and pressures require substantial energy input.
Equipment costs: Thick-walled reactors, compressors, and cooling systems increase capital costs.
Catalyst costs: Some catalysts, such as those containing expensive transition metals, must be used efficiently.
Operating lifetime: Conditions must not degrade equipment or catalysts prematurely.
2. Safety Considerations
Industrial reactions under extreme conditions pose hazards. Safety influences compromise through:
Risk of high-pressure system failure
Handling and storage of hazardous chemicals
Heat management in exothermic processes
Regulatory requirements for safe operation
3. Environmental and Sustainability Factors
Modern industry also considers environmental impact. Operating at excessively high temperatures or pressures increases carbon emissions and energy demand. Optimising conditions contributes to:
Lower energy consumption
Reduced greenhouse gas emissions
Compliance with environmental legislation
Long-term sustainability of catalytic and process resources
Use of Catalysts in Industrial Compromises
Catalysts allow reactions to proceed at lower temperatures by providing an alternative reaction pathway with lower activation energy. This reduces the need for costly or unsafe conditions while maintaining acceptable rates.
Catalyst: A substance that increases reaction rate by providing an alternative pathway with lower activation energy while remaining chemically unchanged.
Catalysts do not affect the equilibrium position, but they help achieve equilibrium faster under milder conditions. This supports economic efficiency and reduces environmental impact.
Industrial catalysts may be:
Heterogeneous: Solid catalysts in contact with gaseous or liquid reactants
Homogeneous: Catalyst and reactants in the same phase
Promoted catalysts: Catalysts enhanced by additives improving activity or lifespan
A sentence before the next definition ensures correct formatting.
Activation Energy: The minimum energy required for a reaction to occur, enabling reactant particles to form an activated complex.
Catalyst choice considers not only activity but also thermal stability, cost, resistance to poisoning, and ease of regeneration.
Recycling and Continuous Processing
Industrial equilibria often reach only partial conversion per pass. To improve yield while maintaining economically reasonable conditions, unreacted reactants are frequently recycled. Advantages include:
Minimising waste
Increasing overall conversion
Allowing operation under more moderate conditions
Reducing raw material costs
Recycling forms part of the broader industrial compromise strategy, allowing equilibrium limitations to be counterbalanced by process engineering solutions.
Summary of Typical Industrial Compromises
Industrial optimisation relies on selecting conditions that meet multiple constraints:
Moderate temperatures to balance yield with rate
Pressures high enough for acceptable yield but low enough for safe, economical operation
Catalyst use to enhance rate under milder conditions
Recycling of reactants to improve overall conversion efficiency
Engineering controls to manage heat flow and maintain stable operating conditions
These compromises fulfil the specification’s emphasis on balancing yield, rate, safety, and economics in industrial equilibrium processes.
FAQ
The chosen temperature must minimise long-term energy expenditure while maintaining throughput. Even if a slightly higher yield could be obtained at a lower temperature, the increased fuel consumption and longer reaction times may outweigh the benefit.
Industrial operators also account for fluctuating energy prices. A temperature that is economically viable during peak energy costs may differ from one chosen during cheaper energy periods.
Catalysts can become poisoned by trace impurities such as sulphur or carbon-containing species, reducing their activity over time.
They may also sinter at high temperatures, leading to a decrease in surface area. Periodic regeneration removes contaminants, while eventual replacement ensures consistent reaction rates without altering equilibrium constraints.
Multiple catalyst beds help maintain optimal temperature profiles. Since exothermic reactions release heat, each bed can be cooled between stages to prevent efficiency loss.
This staging also increases overall conversion per pass by allowing equilibrium to be re-approached gradually without using extreme conditions.
Heat exchangers are built into reactors to remove excess thermal energy from exothermic reactions.
Effective heat management prevents catalyst deactivation, reduces the risk of thermal runaway, and maintains the desired compromise temperature throughout the reactor length.
Impure feedstock can slow reaction rate by poisoning catalysts or introducing side reactions. Improving purity raises efficiency without altering equilibrium.
However, purification steps add cost. Industry balances the advantage of higher reaction rates against the financial and energy costs of additional purification processes.
Practice Questions
Explain why the temperature used in the Haber process is a compromise rather than the lowest possible temperature predicted by equilibrium considerations. (2 marks)
Award up to 2 marks for the following points:
Lowest temperatures give the highest equilibrium yield of ammonia. (1 mark)
However, very low temperatures lead to an unacceptably slow rate of reaction, so a higher temperature is used to balance yield with rate. (1 mark)
In the industrial production of ammonia, several factors must be balanced to achieve an economically viable process. Discuss how temperature, pressure, catalyst use, and recycling of unreacted gases contribute to the overall industrial compromise required for efficient ammonia production.
(5 marks)
Award up to 5 marks for the following points:
Lower temperatures favour the forward exothermic reaction, increasing equilibrium yield, but higher temperatures increase rate. A compromise temperature is used. (1 mark)
Higher pressures increase ammonia yield because fewer gas moles are formed, but very high pressures are costly and pose safety risks. A moderate pressure is chosen. (1 mark)
A catalyst lowers activation energy, allowing acceptable reaction rates at lower temperatures without altering the equilibrium position. (1 mark)
Recycling unreacted nitrogen and hydrogen increases overall conversion without requiring extreme conditions. (1 mark)
Overall compromise must balance yield, rate, energy cost, equipment requirements, and operational safety. (1 mark)

