OCR Specification focus:
‘Classify alcohols as primary, secondary or tertiary based on the number of alkyl groups attached to the carbon bearing the –OH group.’
Alcohols exist in several structural types, and understanding how they are classified is essential because their chemical behaviour depends strongly on substitution around the hydroxyl-bearing carbon.
Classification of Alcohols
What Determines Alcohol Class?
Alcohols are categorised by examining the number of alkyl groups bonded to the carbon atom directly attached to the –OH group. This classification affects their oxidation behaviour, reaction pathways, and stability in many organic processes.
Alcohol functional group: A molecule containing an –OH (hydroxyl) group bonded to a saturated carbon atom.
The class of alcohol depends only on substitution at the carbon carrying the hydroxyl group, not elsewhere in the molecule.
To classify an alcohol, always count how many carbon atoms are attached directly to the OH-bearing carbon.
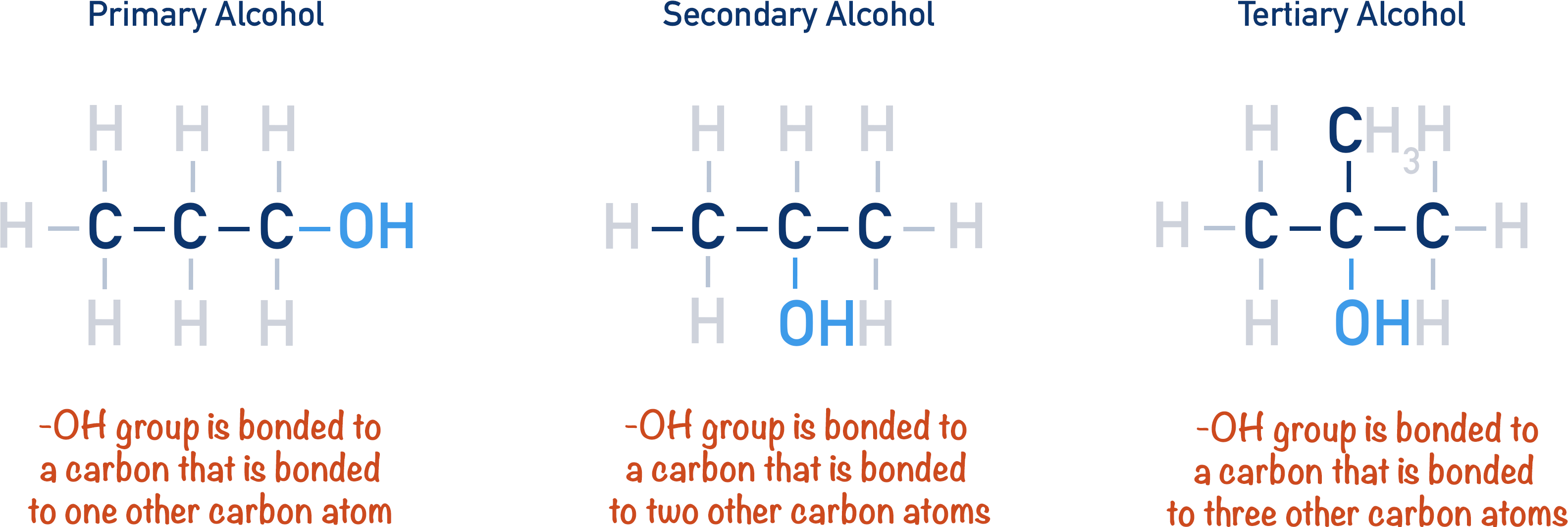
Structures of primary, secondary and tertiary propanols, showing how moving the –OH group changes the classification. The blue-highlighted carbon bears the OH group, with orange text explaining whether it is bonded to one, two, or three carbon atoms. Source
Primary Alcohols (1°)
A primary alcohol contains a hydroxyl group on a carbon attached to one alkyl group and two hydrogens.
Primary alcohol: An alcohol where the carbon bearing the –OH group is bonded to one alkyl group.
Primary alcohols are typically more reactive towards oxidation, but oxidation is beyond the scope of this subsubtopic. Their classification is, however, essential for recognising their role in later reactions.
Primary alcohols may appear as straight-chain structures (e.g. ethanol) or at the end of branched chains.
Secondary Alcohols (2°)
A secondary alcohol has the hydroxyl-bearing carbon attached to two alkyl groups and one hydrogen.
Secondary alcohol: An alcohol where the carbon bearing the –OH group is bonded to two alkyl groups.
Secondary alcohols display reactivity patterns distinct from primary and tertiary structures, and this structural feature influences outcomes in substitution and oxidation reactions discussed elsewhere.
Tertiary Alcohols (3°)
A tertiary alcohol has the –OH group on a carbon attached to three alkyl groups and no hydrogens on that carbon.
Tertiary alcohol: An alcohol where the carbon bearing the –OH group is bonded to three alkyl groups.
The high degree of substitution at this carbon affects both steric hindrance and stability trends encountered in subsequent mechanisms.
In a tertiary alcohol, the OH-bearing carbon is joined to three alkyl groups and no hydrogens, making it the most substituted type.
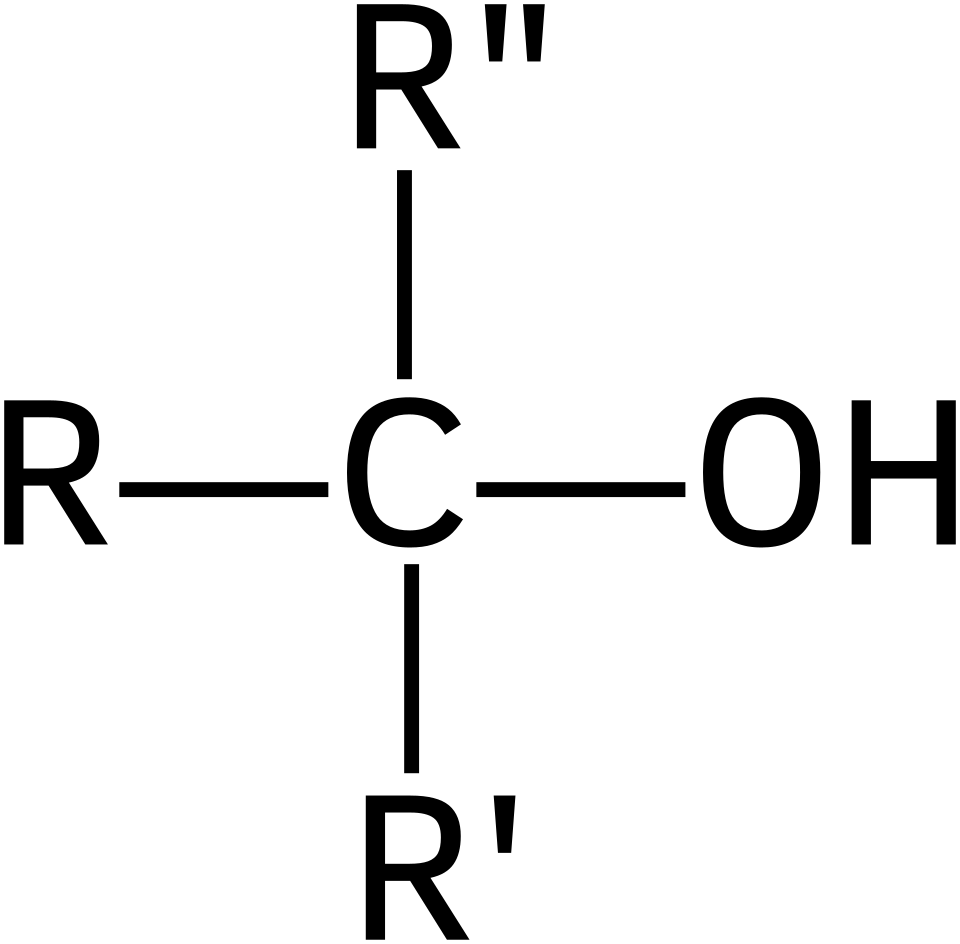
General structure of a tertiary alcohol, showing the OH-bearing carbon attached to three alkyl groups (R₁, R₂, R₃). This highlights that the classification depends solely on substitution at this carbon. The R‑group notation is the same format used throughout A‑Level organic mechanisms. Source
A brief sentence here ensures separation between definition blocks. Tertiary alcohols are structurally bulky, which influences their behaviour in many reactions later in the course.
Importance of Alkyl Substitution
Why Substitution Matters
The number of alkyl groups influences:
Electron-donating ability of surrounding groups
Stability of potential intermediates in later mechanisms
Accessibility of the hydroxyl-bearing carbon to reagents
Patterns of reaction, including oxidation resistance and pathway selection
OCR requires only classification at this stage, but understanding structural trends provides a foundation for future sections on alcohol chemistry.
Structural Identification Strategy
Students should assess substitution systematically:
Locate the –OH group in the structure.
Identify the carbon directly attached to the –OH.
Count the alkyl groups bonded to this carbon.
Classify as primary (one), secondary (two), or tertiary (three).
This approach applies regardless of chain length or complexity.
Structural Variations
Straight-Chain vs Branched Structures
The classification does not depend on the molecule’s total size but strictly on the pattern around the hydroxyl-bearing carbon. For example:
A simple linear alcohol may be primary or secondary depending on where the –OH is located.
Heavily branched molecules often give rise to tertiary alcohols due to multiple alkyl groups converging at a single carbon.
Cyclic Alcohols
Cycloalkanols follow the same classification logic:
If the carbon attached to the –OH has two ring carbons and one hydrogen, it is a secondary alcohol.
A tertiary alcohol can form in a ring system if the hydroxyl carbon is bonded to three carbon atoms within the ring or substituent chains.
Key Structural Features to Observe
Hydrogen Count at the Hydroxyl Carbon
The number of hydrogens attached to the hydroxyl-bearing carbon often provides a quick check:
Primary: two hydrogens
Secondary: one hydrogen
Tertiary: no hydrogens
Nature of Alkyl Groups
Alkyl groups may be:
Straight-chain (e.g. –CH3, –CH2CH3)
Branched chains
Ring carbons
All count equally when determining substitution level.
Misconceptions to Avoid
Students sometimes misclassify alcohols because:
They count alkyl groups elsewhere in the molecule rather than only those attached to the hydroxyl carbon.
They confuse the total number of carbons with the degree of substitution.
They misidentify the hydroxyl carbon in complex structures.
Focusing strictly on the carbon directly bonded to the –OH group avoids these errors.
Applying the OCR Requirement
What OCR Expects You to Know
OCR requires you to:
Correctly classify alcohols as primary, secondary, or tertiary
Base classification solely on the number of alkyl groups attached to the carbon bearing the –OH group
Recognise that classification affects later reactivity, although those reactions are not part of this subsubtopic
Practical Tips for Exam Context
When analysing structural formulae:
Circle the hydroxyl carbon before counting attached groups.
Check for hidden hydrogens on skeletal formulae.
Remember that the classification system is purely structural, not functional beyond identification.
This systematic method ensures reliable and consistent classification in all alcohol structures encountered in OCR A-Level Chemistry.
FAQ
The OH group’s position determines how many alkyl groups surround the carbon bearing it.
If it lies at the end of a chain, the alcohol is usually primary.
If it lies on a more substituted internal carbon, the alcohol is secondary or tertiary depending on how many carbon chains branch from that point.
This means structural location alone is not enough; the immediate bonding environment of the OH-bearing carbon must always be examined directly.
In a tertiary alcohol, the carbon bonded to the OH group forms single bonds with three alkyl groups.
Because carbon can only form four bonds, no hydrogens remain attached.
This structural feature contributes to steric hindrance and influences reaction pathways studied in later subtopics.
Skeletal formulae omit hydrogens bonded to carbons, so students must mentally add them to determine substitution.
Misidentification often occurs when the OH-bearing carbon looks similar to neighbouring carbons.
To classify correctly:
Locate the carbon directly bonded to the OH.
Visualise or draw out all implied hydrogens.
Count only the carbon chains attached to this carbon.
Yes, but only when the OH-bearing carbon is bonded to one ring carbon and one hydrogen.
Most simple cycloalkanols are secondary because the OH-bearing carbon is usually attached to two neighbouring ring carbons.
Primary cyclic alcohols appear when the OH group is located on a carbon at the end of a ring substituent rather than on the ring itself.
Hydrogen counts can become ambiguous in complex or branched structures, whereas alkyl groups remain consistently identifiable as carbon-containing substituents.
Counting alkyl groups ensures correct classification because:
Each alkyl group denotes a carbon chain bonded to the OH-bearing carbon.
Substitution level depends solely on these carbon–carbon attachments.
Skeletal formulas with implied hydrogens do not change the number of alkyl groups present.
Practice Questions
Butan-2-ol and 2-methylpropan-1-ol are both structural isomers with the formula C4H10O.
(a) State the classification of each alcohol as primary, secondary, or tertiary.
(b) Explain your reasoning in each case.
(3 marks)
(a)
Butan-2-ol: secondary alcohol (1 mark)
2-methylpropan-1-ol: primary alcohol (1 mark)
(b)
Butan-2-ol has the OH-bearing carbon attached to two alkyl groups. (1 mark)
2-methylpropan-1-ol has the OH-bearing carbon attached to one alkyl group. (1 mark, if explanation clearly linked)
(Max 3 marks total)
Figure 1 shows the skeletal formula of an unknown alcohol.
Using the structural information, answer the following:
(a) Identify the carbon that bears the OH group.
(b) Determine whether the alcohol is primary, secondary, or tertiary.
(c) Describe how the number of alkyl groups attached to this carbon leads to your classification.
(d) State one common misconception students make when classifying alcohols and explain why it is incorrect.
(5 marks)
(a)
Correct identification of the OH-bearing carbon (e.g. description matching diagram, such as “the carbon directly bonded to the OH group”). (1 mark)
(b)
Correct classification as primary / secondary / tertiary, depending on the structure provided. (1 mark)
(c)
Clear explanation that classification depends on counting the alkyl groups attached to the OH-bearing carbon only. (1 mark)
Correct justification using “one alkyl group = primary”, “two = secondary”, or “three = tertiary”. (1 mark)
(d)
States a valid misconception, such as “counting total carbons in the molecule” or “counting groups not attached to the OH carbon”. (1 mark)
Explains why it is incorrect, referring to the correct classification method. (1 mark)
(Max 5 marks total)

