OCR Specification focus:
‘Describe combustion of alcohols, comparing energy release and typical products with analogous alkane combustion where relevant.’
Alcohol combustion is a key reaction in organic chemistry, demonstrating how molecular structure influences energy release and the identity of reaction products when burned in oxygen-containing environments.
Combustion of Alcohols
Alcohols undergo combustion when heated in the presence of sufficient oxygen, forming carbon dioxide and water as the primary products.

Ethanol burning on a shallow dish, producing a clean blue flame under conditions of complete combustion. The flame represents alcohol molecules reacting with oxygen to form carbon dioxide and water, releasing heat energy. Although this photograph does not show products directly, it visually reinforces the appearance of alcohol combustion discussed in the notes. Source
Complete Combustion
In complete combustion, an alcohol burns in an excess supply of oxygen.
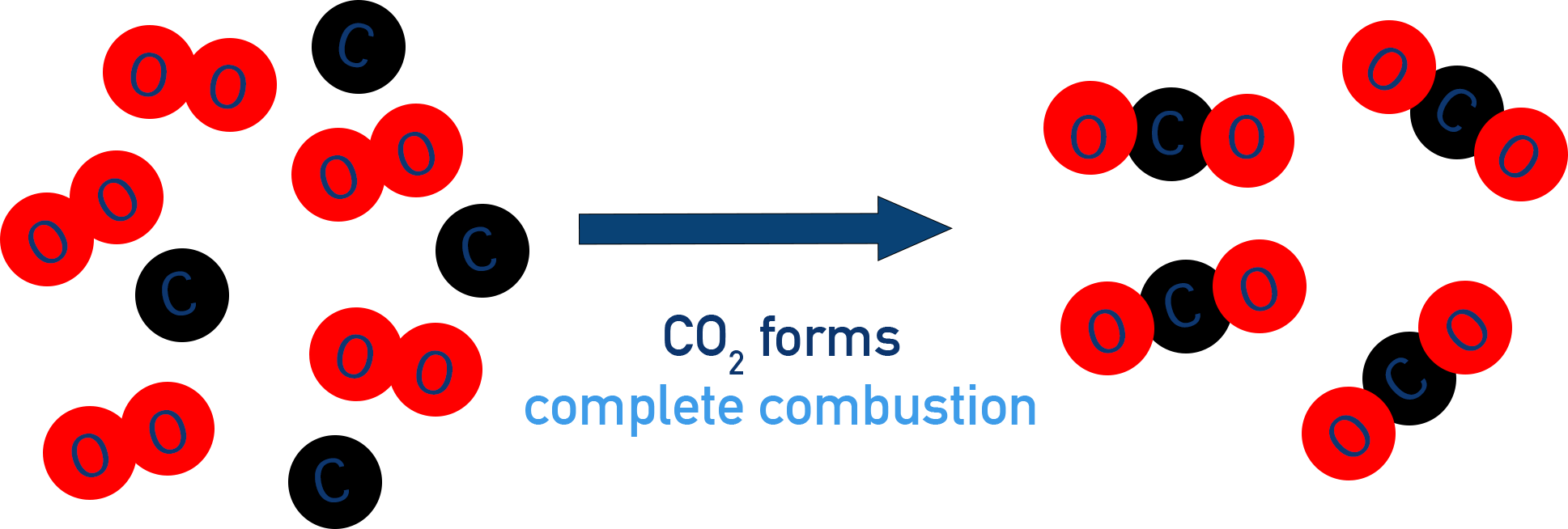
Molecular-level diagram of complete combustion, where each carbon atom combines with sufficient oxygen to form carbon dioxide. The left side shows separate oxygen and carbon species, while the right side shows only CO₂ molecules after combustion. Although drawn for hydrocarbons in general, the same idea of full oxidation to CO₂ applies to alcohol fuels such as ethanol. Source
Products are predictable because the functional group and hydrocarbon chain oxidise fully to the highest possible oxidation states of carbon and hydrogen.
Alcohol + excess O₂ → CO₂ + H₂O
Produces a clean, blue flame (typical for small alcohols such as methanol and ethanol)
Releases significant heat energy due to extensive bond formation
Complete Combustion of Ethanol: C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O
C₂H₅OH = Ethanol, the combusting fuel
O₂ = Oxygen required for complete oxidation
CO₂ = Carbon dioxide produced
H₂O = Water produced
Complete combustion depends on adequate oxygen supply. Larger alcohols still combust cleanly when oxygen is abundant, though their flames may appear more orange due to incomplete vapour mixing.
Incomplete Combustion
Incomplete combustion occurs when oxygen availability is limited, preventing full oxidation.
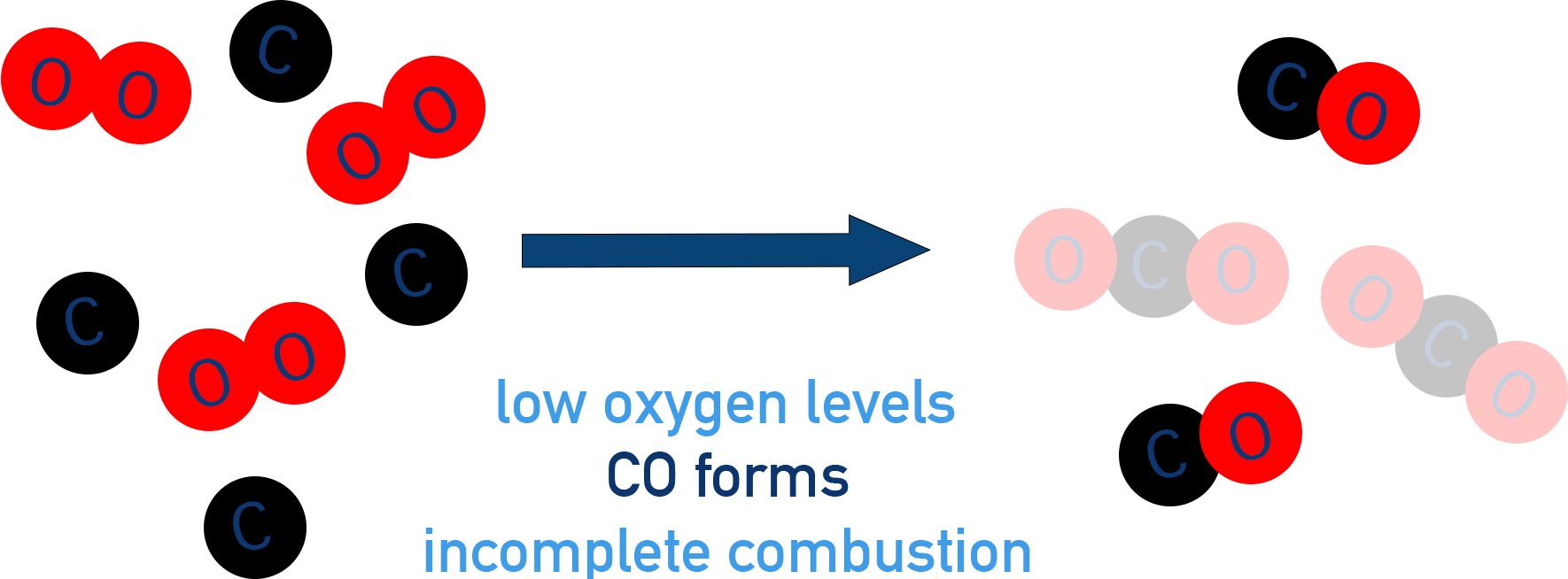
Diagram showing how low oxygen levels lead to incomplete combustion, with carbon monoxide molecules forming instead of full conversion to carbon dioxide. The left side depicts separate oxygen and carbon species, while the right side shows CO molecules as the main carbon-containing product. Although drawn in a general way for hydrocarbon fuels, the same principle applies to alcohols when they burn in an oxygen-poor environment. Source
Produces carbon monoxide (CO), carbon (soot), and water
Produces a yellow, smoky flame
Releases less energy than complete combustion
Carbon Monoxide: A toxic gas formed when carbon undergoes partial oxidation during insufficient oxygen supply.
Incomplete combustion is common for longer-chain alcohols or when air supply is restricted (e.g., poorly ventilated burners). This reduced efficiency is important in evaluating alcohols as fuels.
A normal sentence must occur here to ensure appropriate spacing before the next definition block.
Exothermic Reaction: A process that releases heat energy to the surroundings due to overall stronger bond formation in the products than in the reactants.
Comparing Alcohols with Alkanes
The OCR specification requires students to compare energy release and combustion products of alcohols to those of analogous alkanes. Both homologous series combust exothermically, but their molecular structures affect the overall energy profile.
Key comparison points:
Alcohols contain an O–H group, which introduces polarity not present in alkanes.
Alcohols typically exhibit lower volatility than alkanes of similar size because hydrogen bonding reduces evaporation rate.
Despite this, both alcohols and alkanes produce CO₂ and H₂O under complete combustion.
Alcohols generally release slightly less energy per mole than corresponding alkanes because the alcohol molecule is already partially oxidised due to its oxygen content.
Trends in Combustion Energy
The amount of heat released in combustion increases with carbon chain length. This applies to both alcohols and alkanes, but alcohol combustion must account for the presence of oxygen within the molecular structure.
Bullet-point trends:
Heat released increases with molecular size (more C–H and C–C bonds available for oxidation).
Alcohols produce less heat than alkanes with the same number of carbon atoms.
Primary, secondary, and tertiary alcohols all undergo combustion; classification does not significantly change combustion pathways, as all are fully oxidised to CO₂ and H₂O under complete combustion conditions.
Physical Properties Relevant to Combustion
The OCR specification highlights comparisons to alkanes. Alcohols differ due to polarity and hydrogen bonding, both of which influence combustion behaviour.
Polarity: Uneven distribution of electron density in a bond or molecule, arising from differences in electronegativity.
Hydrogen bonding increases boiling points, making alcohols less volatile than alkanes, which can influence their burning characteristics—particularly ignition temperature and flame stability.
Practical Observations in Alcohol Combustion
Students should be familiar with typical laboratory observations when burning alcohols:
Smaller alcohols (methanol, ethanol, propanol) ignite easily with a clear flame.
Larger alcohols may burn with less consistent flames due to lower volatility.
Alcohol burners rely on the wick drawing liquid fuel upwards, meaning vapour production affects combustion efficiency.
Combustion Products and Environmental Considerations
Although the OCR specification for this subsubtopic does not emphasise environmental chemistry, it remains essential to understand the implications of combustion products when comparing alcohols and alkanes.
Key points:
Complete combustion → CO₂ + H₂O; contributes to greenhouse gas output.
Incomplete combustion → CO + C (soot); CO is highly toxic and soot contributes to particulate pollution.
Alcohols can be considered cleaner-burning than alkanes owing to a tendency to produce fewer particulates when fully combusted.
Overall, understanding alcohol combustion requires recalling core ideas from redox chemistry, energy changes, and intermolecular forces while focusing on the OCR specification requirement to compare combustion behaviour and energy release with analogous alkanes.
FAQ
Smaller alcohols ignite more readily because they have higher volatility, allowing them to form flammable vapours quickly. As chain length increases, volatility decreases due to stronger intermolecular forces, requiring higher temperatures to achieve ignition.
Branching also increases volatility slightly, meaning branched alcohols may ignite more easily than their straight-chain isomers.
Soot formation increases as carbon chain length increases. Larger alcohols contain a greater proportion of carbon atoms, making complete oxidation more difficult when oxygen is limited.
Under restricted airflow, longer-chain alcohols produce more solid carbon particles because their combustion pathways favour partial oxidation and carbon deposition.
Spirit burners provide a controlled, steady flame and allow easy measurement of fuel mass before and after burning. This makes them practical for comparing enthalpy changes between different alcohols.
However, heat loss to the surroundings, incomplete combustion, and evaporation of the alcohol can cause experimental values to be lower than theoretical values.
Hydrogen bonding increases boiling points, meaning alcohols evaporate more slowly than alkanes. This reduces vapour production, causing slower flame propagation.
In practical terms, fuels that evaporate more slowly:
Ignite less readily
Burn with less intensity
May require wicks or pre-heating for sustained combustion
Smaller alcohols typically burn with a blue flame because they evaporate efficiently and mix well with oxygen, favouring complete combustion.
Larger alcohols evaporate less readily, leading to poorer oxygen mixing. This encourages incomplete combustion, producing yellow or orange flames due to glowing carbon particles (soot) in the flame.
Practice Questions
Ethanol undergoes complete combustion when burned in an excess of oxygen.
(a) State the two products formed in the complete combustion of ethanol.
(b) Explain why the combustion of ethanol is described as exothermic.
(2 marks)
(a)
Carbon dioxide stated. (1)
Water stated. (1)
(b)
Reference to heat energy being released to the surroundings / negative enthalpy change. (1)
(Max 2 marks total)
An alcohol and an alkane of the same carbon chain length are burned separately under identical conditions.
(a) Explain why the alcohol releases less energy per mole than the alkane when completely combusted.
(b) Describe what happens when the alcohol undergoes incomplete combustion and state two possible products formed.
(c) Suggest one visual observation that would indicate incomplete combustion.
(5 marks)
(a)
Alcohol already partially oxidised because it contains an oxygen atom in its structure. (1)
Therefore less energy released on combustion compared with the fully reduced alkane. (1)
(b)
Incomplete combustion occurs when insufficient oxygen is present. (1)
Carbon monoxide formed. (1)
Carbon/soot may also form. (1)
(c)
Yellow/orange smoky flame stated. (1)
(Max 5 marks total)

