OCR Specification focus:
‘Use acidified dichromate(VI) to oxidise: primary alcohols to aldehydes or further to carboxylic acids under different conditions; secondary alcohols to ketones; tertiary resist oxidation.’
Introduction
Alcohol oxidation is a key transformation in organic chemistry, allowing controlled conversion of primary alcohols, secondary alcohols, and resistance in tertiary alcohols to be understood using specific reagents and conditions.
Oxidation of Alcohols: Core Principles
Oxidation of alcohols involves increasing the number of carbon–oxygen bonds in a molecule. In OCR Chemistry, oxidation is carried out using acidified dichromate(VI), typically K₂Cr₂O₇/H₂SO₄, which changes colour from orange to green during reduction. This visual indicator helps monitor progression during heating.
When an alcohol reacts with this oxidising agent, the products formed depend entirely on the alcohol’s classification and the reaction conditions employed. These transformations are foundational to understanding reaction pathways in organic synthesis.
Oxidation (organic): The chemical process in which a molecule increases its proportion of oxygen bonds, often through the gain of oxygen or loss of hydrogen.
Aldehydes, ketones and carboxylic acids differ in structure, reactivity and formation pathways, making the control of reaction conditions essential.
Primary Alcohols: Formation of Aldehydes and Carboxylic Acids
Primary alcohols can undergo partial or complete oxidation, depending on how strongly the reaction is driven.
Partial Oxidation: Forming Aldehydes
To obtain an aldehyde, conditions must prevent further oxidation. This requires:
Using acidified dichromate(VI) as the oxidising agent
Gentle heating, often with distillation as the alcohol oxidises
Immediate removal of the aldehyde from the hot mixture to avoid further oxidation
The functional group produced is the –CHO (aldehyde) group, which is highly reactive.
Complete Oxidation: Forming Carboxylic Acids
Primary alcohols can be pushed further to produce carboxylic acids, containing the –COOH functional group. This occurs when oxidation conditions encourage continuous reaction.
Heat strongly under reflux, ensuring the alcohol remains in contact with the oxidising agent
Use excess dichromate(VI) to maintain an oxidising environment
Reflux prevents escape of volatile aldehydes, allowing complete oxidation
These two outcomes exemplify how reaction control leads to different functional groups through the same reagent.
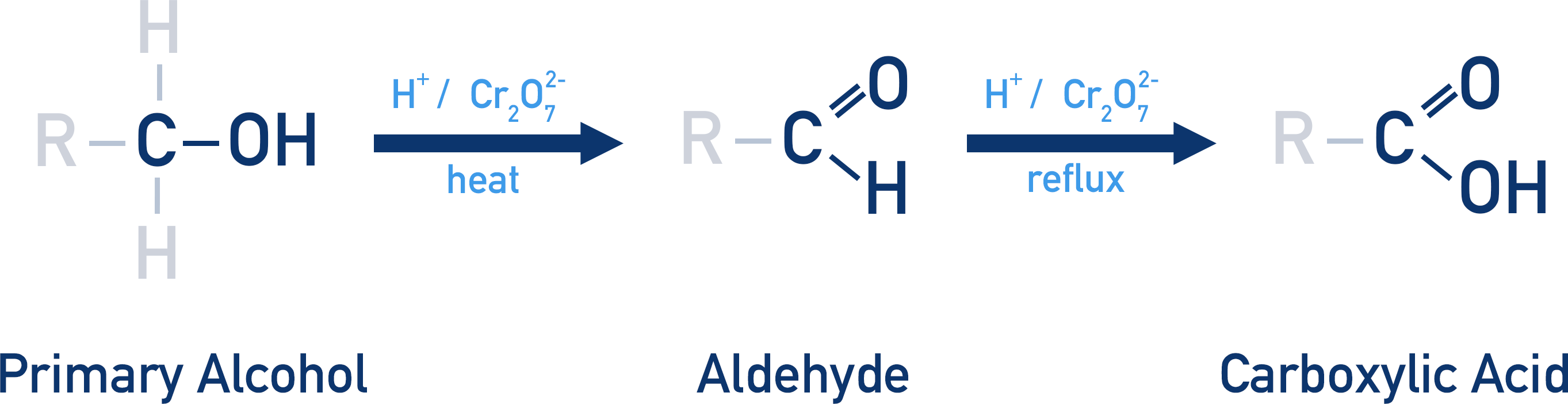
This diagram illustrates the oxidation pathway of a primary alcohol using H⁺/Cr₂O₇²⁻, showing aldehyde formation under distillation and carboxylic acid formation under reflux. It reinforces how reaction conditions determine product outcome. All details are relevant to the OCR A-Level specification. Source
Secondary Alcohols: Formation of Ketones
Secondary alcohols oxidise to form ketones, characterised by a C=O group bonded to two carbon atoms. Unlike aldehydes, ketones are resistant to further oxidation under standard conditions.
Reflux with acidified dichromate(VI) is typically used
Only one oxidation step occurs
No further oxidation to carboxylic acids takes place
Ketones are therefore stable endpoints for secondary alcohol oxidation within this syllabus.
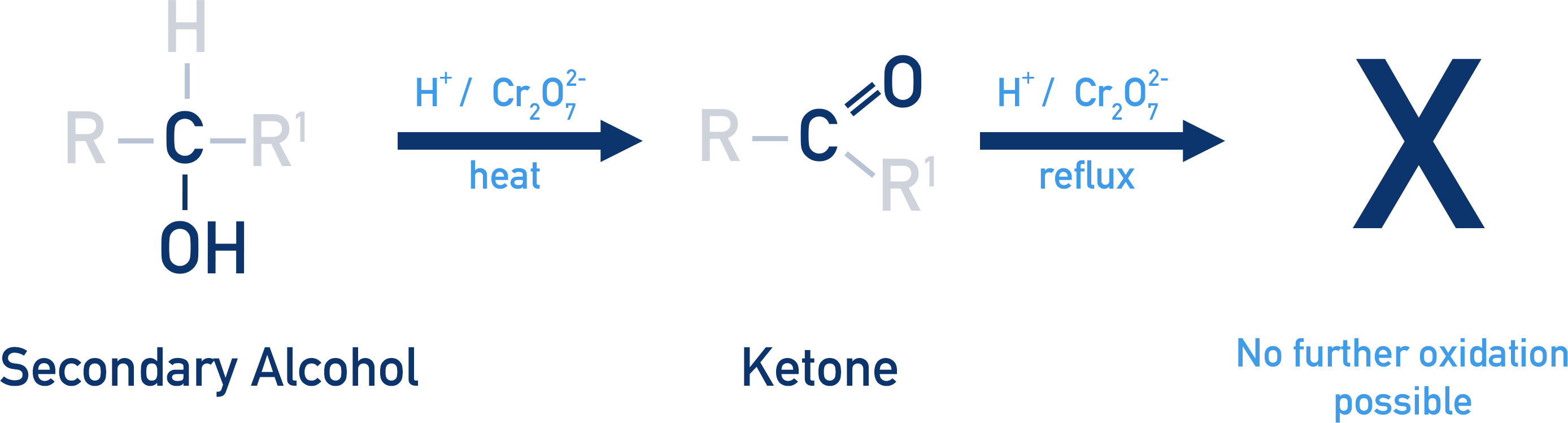
This image shows a secondary alcohol oxidised by H⁺/Cr₂O₇²⁻ to form a ketone, with no further reaction occurring under reflux. It highlights the stability of ketones as final oxidation products, as required by OCR A-Level Chemistry. Source
Ketone: An organic compound featuring a carbonyl group (C=O) bonded to two carbon atoms.
This stability is important when identifying oxidation pathways and predicting reaction outcomes.
Tertiary Alcohols: Resistance to Oxidation
Tertiary alcohols do not oxidise using acidified dichromate(VI). This is due to the absence of a hydrogen atom on the carbon carrying the –OH group, which prevents the required removal of hydrogen during oxidation.
No colour change from orange to green is observed
Heating, refluxing or increasing oxidant concentration has no effect
Stronger oxidising methods beyond the A-Level syllabus would be required to break C–C bonds
Tertiary alcohol: An alcohol in which the carbon bearing the –OH group is bonded to three alkyl groups, leaving no hydrogen available for oxidation.
Tertiary alcohol behaviour provides a useful diagnostic tool when studying reaction pathways.
Reflux and Distillation in Oxidation Processes
Control over oxidation products relies on appropriate apparatus use:
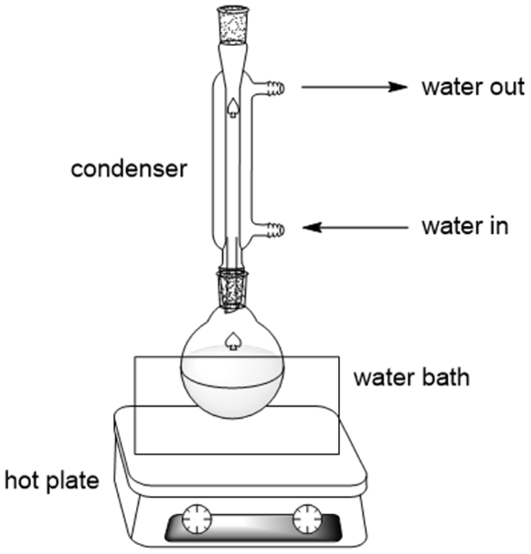
This diagram depicts a typical reflux setup, including condenser water flow and heating arrangement. It helps students visualise the laboratory method used for complete oxidation of primary alcohols and oxidation of secondary alcohols. Extra procedural detail is included but remains consistent with OCR expectations. Source
Distillation
Used to obtain aldehydes from primary alcohols.
Allows aldehyde to vaporise and condense away from the oxidising mixture
Prevents further oxidation
Minimises exposure to heat
Reflux
Used when complete oxidation is required or when secondary alcohols form ketones.
Ensures continuous heating without loss of volatile substances
Returns vapours to the reaction flask, promoting full oxidation
Maintains reaction consistency and safety
These techniques support the manipulation of oxidation levels during synthesis.
Key Observations in Alcohol Oxidation
Students should recognise several important experimental features:
Colour change: orange dichromate(VI) → green chromium(III)
Odour differences: aldehydes often have distinctive smells
Boiling point changes: aldehydes and ketones exhibit lower boiling points than their alcohol precursors
Functional group formation: aldehydes (–CHO), ketones (C=O), carboxylic acids (–COOH)
These observations strengthen the understanding of oxidation as a controlled chemical transformation.
Summary of Oxidation Pathways
Primary alcohol
Gentle heating/distillation → aldehyde
Reflux/excess oxidant → carboxylic acid
Secondary alcohol
Reflux → ketone only
Tertiary alcohol
No oxidation with dichromate(VI)
Mastery of these pathways is essential for predicting products and planning organic syntheses within the OCR A-Level Chemistry framework.
FAQ
The ease of oxidation depends on whether the carbon bonded to the OH group has an attached hydrogen atom.
Primary and secondary alcohols possess this hydrogen, enabling oxidation through the removal of hydrogen atoms.
Tertiary alcohols lack such a hydrogen, so oxidation would require breaking a carbon–carbon bond, which dichromate(VI) cannot achieve under standard laboratory conditions.
Acidified dichromate(VI) offers a clear, visible colour change from orange to green, allowing students to monitor oxidation easily.
Its strength and reliability mean it consistently oxidises primary alcohols to aldehydes or acids and secondary alcohols to ketones.
Other oxidising agents may be less predictable, lack a colour change, or produce unwanted side-products under comparable conditions.
The likelihood of further oxidation depends on:
Reaction temperature
Concentration of oxidising agent
Whether the aldehyde remains in contact with the oxidant
Distillation prevents prolonged contact, while reflux keeps all substances together and encourages full oxidation to the carboxylic acid.
Aldehydes contain a hydrogen atom on the carbonyl carbon, making them more susceptible to oxidation.
Ketones lack this hydrogen and require stronger oxidising agents to undergo further change.
Additionally, steric hindrance around the carbonyl group is typically greater in ketones, further reducing their reactivity.
Aldehydes often have low boiling points and can evaporate easily, making them difficult to collect efficiently.
They also oxidise readily, so any delay in removal increases the risk of forming a carboxylic acid instead.
Students must carefully control heating, distillation rate, and cooling to achieve good yields.
Practice Questions
A student heats a primary alcohol with acidified potassium dichromate(VI) under distillation conditions.
State the organic product formed and explain why distillation is used.
(2 marks)
1 mark – Identifies the product as an aldehyde.
1 mark – Explains that distillation prevents further oxidation by removing the aldehyde from the reaction mixture.
A secondary alcohol and a tertiary alcohol are each heated separately under reflux with excess acidified potassium dichromate(VI).
(a) State the expected organic product formed from the secondary alcohol.
(b) Explain why the tertiary alcohol does not undergo oxidation under these conditions.
(c) Describe two visible observations that could help confirm whether oxidation has occurred.
(5 marks)
(a)
1 mark – States that the secondary alcohol forms a ketone.
(b)
Up to 2 marks:
States that tertiary alcohols lack a hydrogen atom on the carbon bonded to the OH group (1 mark).
Therefore oxidation cannot occur without breaking C–C bonds, which the reagent cannot do under these conditions (1 mark).
(c)
Up to 2 marks:
Orange dichromate(VI) solution turns green (1 mark).
Additional valid observation such as characteristic ketone smell not produced for tertiary alcohols or lack of temperature change for the tertiary sample (1 mark).

