OCR Specification focus:
‘Explain polarity of alcohols; hydrogen bonding accounts for higher water solubility and lower volatility than alkanes of similar size.’
Alcohols show distinctive physical properties due to their polarity and ability to form hydrogen bonds. Understanding these interactions helps explain their solubility behaviour, boiling points, and trends compared with analogous alkanes.
Polarity of Alcohols
Alcohols contain an O–H group, which introduces significant polarity into the molecule. This arises because oxygen is far more electronegative than hydrogen and carbon, creating unequal electron distribution across the bond.
Polarity: The uneven distribution of electron density within a covalent bond, resulting in a partial positive charge on one atom and a partial negative charge on another.
The electronegative oxygen atom draws bonding electrons towards itself, giving it a partial negative charge (δ–). The hydrogen and carbon atoms bonded to oxygen therefore carry partial positive charges (δ+). This bond polarity influences the way alcohol molecules interact with each other and with surrounding substances, particularly water.
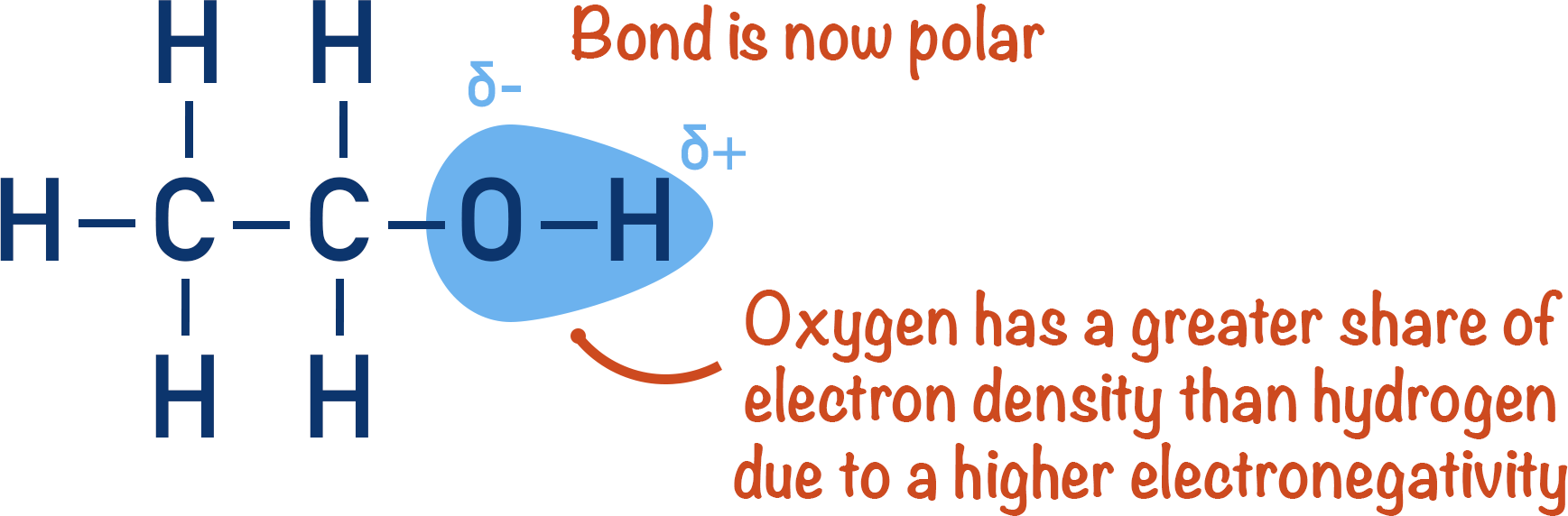
This diagram shows ethanol with its polar O–H bond, highlighting electron density around oxygen and illustrating how the hydroxyl group contributes to molecular polarity. Source
Key Features of Alcohol Polarity
The O–H bond is highly polar.
The C–O bond is also polar, contributing to overall molecular polarity.
Short-chain alcohols (e.g., methanol, ethanol) have a dominant polar region relative to their hydrocarbon chain.
Longer-chain alcohols become progressively less polar overall as the non-polar alkyl chain increases in size.
These effects help explain observable property trends in solubility and volatility.
Hydrogen Bonding in Alcohols
Hydrogen bonding is a specific type of strong intermolecular force that arises between molecules containing a hydrogen atom bonded to a highly electronegative atom such as oxygen.
Hydrogen Bonding: A strong intermolecular attraction between a δ+ hydrogen atom bonded to O, N or F and a lone pair on an electronegative atom in another molecule.
Hydrogen bonding plays a central role in the physical behaviour of alcohols. When two alcohol molecules interact, the hydrogen of the O–H group in one molecule can attract the lone pair on the oxygen of another. This creates a network of intermolecular forces that are significantly stronger than the London dispersion forces present in corresponding alkanes.
A normal sentence placed here ensures appropriate spacing before any other structured block appears.
Why Hydrogen Bonding Matters
Alcohols can:
Hydrogen bond with themselves (intermolecular hydrogen bonding).
Hydrogen bond with water, leading to enhanced solubility for smaller alcohols.
Exhibit higher boiling points and lower volatility than alkanes of similar molar mass.
These properties stem directly from the energy required to overcome hydrogen bonds during phase changes such as boiling or evaporation.
Solubility of Alcohols in Water
The ability of alcohols to mix with water depends largely on the strength and extent of hydrogen bonding that can occur between the molecules. Water, being highly polar and capable of forming extensive hydrogen bonding networks, forms strong attractions with the O–H group of alcohols.
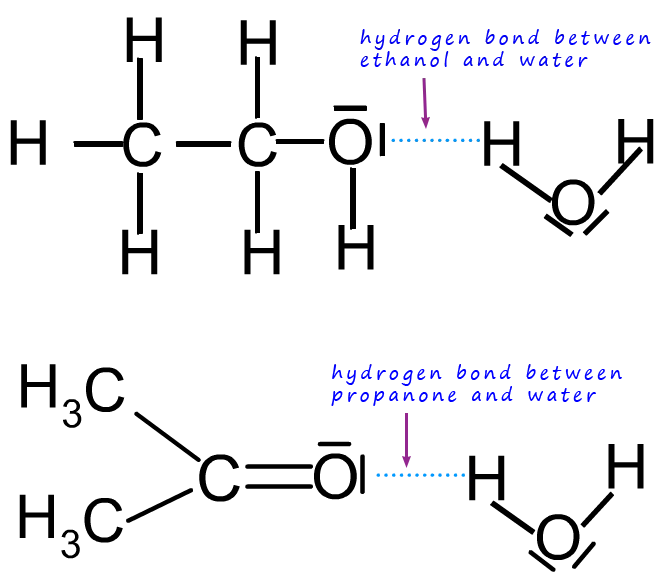
The upper diagram shows hydrogen bonding between ethanol and water, demonstrating why short-chain alcohols dissolve readily. The lower propanone example extends the principle but exceeds the required syllabus focus. Source
Factors Affecting Solubility
Short-chain alcohols (e.g., methanol, ethanol, propan-1-ol):
Highly soluble in water.
Their polar O–H group dominates the molecule.
Hydrogen bonds form readily with water molecules.
Long-chain alcohols (e.g., pentan-1-ol or higher):
Decreasing solubility as chain length increases.
The non-polar hydrocarbon chain interferes with water’s hydrogen-bonded network.
Solubility becomes limited because less of the molecule can engage in strong intermolecular bonding with water.
Specification Link
The OCR specification emphasises that hydrogen bonding accounts for the higher water solubility of alcohols relative to analogous alkanes. Alkanes are non-polar and cannot form hydrogen bonds, so they are insoluble in water.
Volatility and Boiling Point Trends
Volatility refers to how readily a liquid vapourises. Boiling point is directly linked to the strength of intermolecular forces: stronger forces require more energy to overcome.
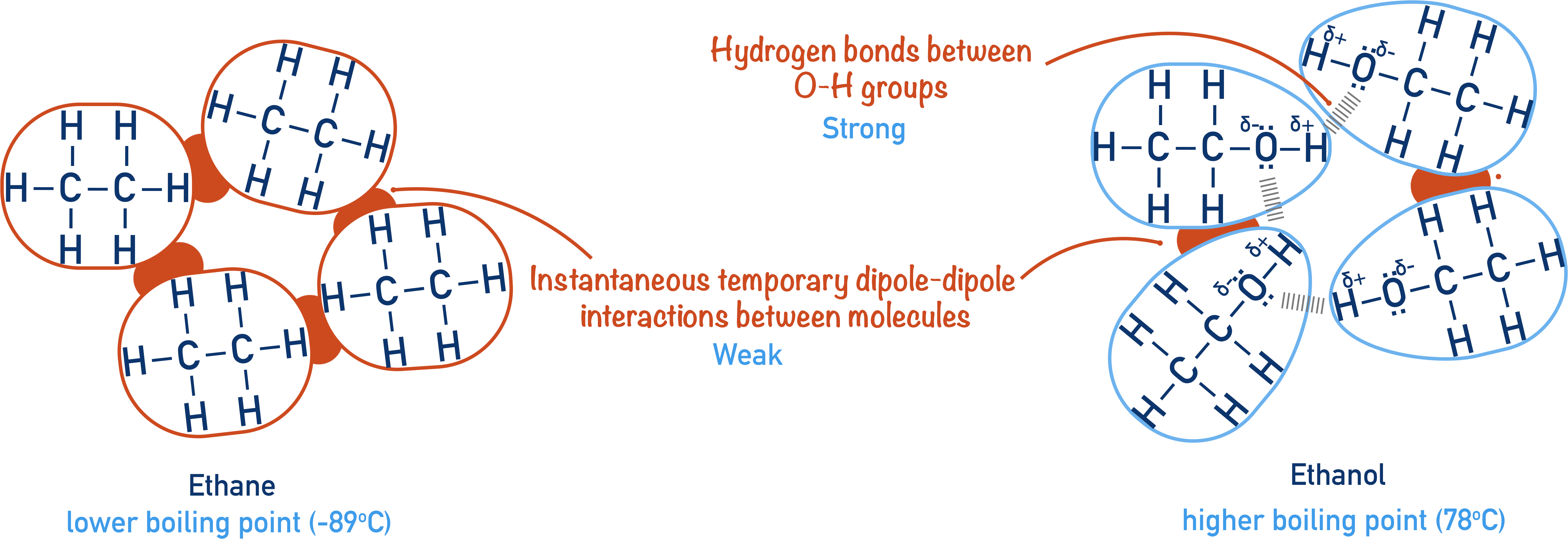
This comparison illustrates why ethanol’s hydrogen bonding results in a significantly higher boiling point than ethane, which relies solely on weak dispersion forces. Source
Why Alcohols Have Lower Volatility than Alkanes
Alcohols contain hydrogen bonds, which:
Require significantly more energy to break than London dispersion forces alone.
Lead to higher boiling points compared with alkanes of similar relative molecular mass.
Produce lower volatility because fewer molecules escape into the vapour phase at a given temperature.
Boiling Point Patterns Within Alcohols
Increasing chain length increases London dispersion forces, raising boiling points.
Position of the O–H group remains the principal reason alcohols deviate markedly from alkanes in boiling behaviour.
Branched alcohols tend to have lower boiling points than straight-chain isomers because branching reduces surface contact and weakens dispersion forces, even though hydrogen bonding remains present.
Comparing Alcohols and Alkanes
The specification highlights the contrast between these two homologous series. Key differences include:
Intermolecular Forces
Alcohols: Hydrogen bonding + dispersion forces + dipole–dipole interactions.
Alkanes: Only dispersion forces.
Resulting Physical Properties
Alcohols have higher boiling points.
Alcohols show greater solubility in water.
Alcohols demonstrate lower volatility.
Why This Matters Chemically
Differences in intermolecular forces influence how alcohols behave in reactions requiring heating.
Physical properties affect purification methods such as distillation.
Solubility trends help predict reaction environments, especially for aqueous oxidations or substitutions.
Summary of OCR Learning Points
Although not a conclusion, the key specification requirements are embedded throughout this page:
Alcohols are polar molecules due to the electronegativity of oxygen.
Hydrogen bonding explains their solubility in water and relatively low volatility.
These features distinguish alcohols clearly from alkanes of similar size.
FAQ
Alcohols contain a polar O–H group and a non-polar hydrocarbon chain, giving them dual character.
This means their solubility depends on the balance between these regions.
Short-chain alcohols are mostly polar and dissolve poorly in non-polar solvents.
Longer-chain alcohols dissolve more readily because the hydrocarbon region dominates, allowing stronger London dispersion interactions with non-polar molecules.
Branching reduces the surface area of the hydrocarbon portion of the molecule.
This leads to:
Weaker London dispersion forces
Less efficient molecular packing
Even though the O–H group can still form hydrogen bonds, the overall intermolecular attractions weaken, resulting in a lower boiling point.
The primary factor is the presence of the O–H group, which can donate one hydrogen bond and accept two through its lone pairs.
Other influences include:
Steric hindrance around the hydroxyl group
Molecular shape, affecting how closely molecules can approach
Competing interactions, such as strong intramolecular attractions in some substituted alcohols
Volatility is governed by the type and strength of intermolecular forces present.
If two alcohols have the same mass but different structures:
One may experience greater hydrogen bonding due to less steric hindrance
One may have stronger dispersion forces due to a more extended carbon chain
Differences in branching can also modify how effectively molecules interact
These structural variations alter the energy needed for molecules to evaporate.
Hydrogen bonds weaken as temperature increases because molecules gain kinetic energy.
This leads to:
Reduced overall hydrogen-bond density
Increased molecular motion disrupting ordered bonding networks
Slightly lower miscibility for some longer-chain alcohols at higher temperatures
However, for short-chain alcohols, complete miscibility remains across typical temperature ranges.
Practice Questions
Explain why ethanol has a higher boiling point than ethane.
(2 marks)
1 mark: Identifies that ethanol can form hydrogen bonds between its molecules.
1 mark: States that hydrogen bonds require more energy to overcome than the London dispersion forces in ethane.
Ethanol is highly soluble in water, whereas hexan-1-ol is only sparingly soluble.
Using your knowledge of intermolecular forces, explain the difference in solubility between these two alcohols.
In your answer, refer to both hydrogen bonding and the effect of increasing hydrocarbon chain length.
(5 marks)
1 mark: States that ethanol forms hydrogen bonds with water due to its polar O–H group.
1 mark: Explains that these hydrogen bonds allow ethanol to dissolve readily in water.
1 mark: States that hexan-1-ol has a much larger non-polar hydrocarbon chain.
1 mark: Explains that the increasing non-polar proportion reduces the molecule’s ability to hydrogen bond with water.
1 mark: Concludes that the large hydrophobic chain disrupts water’s hydrogen-bonding network, making hexan-1-ol only sparingly soluble.

