OCR Specification focus:
‘Eliminate water from alcohols using acid catalyst (H3PO4 or H2SO4) and heat to form alkenes; mechanism not required.’
Alcohol dehydration is a key transformation in organic chemistry, enabling the conversion of alcohols into alkenes using acidic conditions and heat. This reaction illustrates how functional groups can be manipulated to create new structures with different reactivity patterns, an essential skill in synthetic chemistry.
Dehydration of Alcohols to Form Alkenes
Dehydration refers to the elimination of water from a molecule. When applied to alcohols, this process converts an alcohol into an alkene, a functional group characterised by a carbon–carbon double bond. This transformation is widely used in synthetic chemistry to introduce unsaturation into molecules.
The diagram shows ethanol undergoing dehydration with concentrated sulfuric acid and heat to form ethene and water, illustrating the elimination of H and OH to create the double bond. Source
Alcohol dehydration: The elimination of water from an alcohol to form an alkene under acidic conditions and heat.
This reaction is classed as an elimination reaction, meaning atoms or groups are removed from adjacent carbon atoms, creating a double bond.
Conditions Required for Dehydration
The OCR specification emphasises the need for an acid catalyst and heat. Suitable catalysts include phosphoric acid (H₃PO₄) and sulfuric acid (H₂SO₄), both of which act by protonating the alcohol to make the –OH group easier to remove.
Typical Conditions
Concentrated acid catalyst: Usually H₃PO₄ or H₂SO₄.
Heating: Higher temperatures favour elimination.
Alcohol in liquid phase: Most commonly applied to primary, secondary, and tertiary alcohols.
Distillation or reflux: Distillation is often used to remove the alkene as it forms, shifting equilibrium towards the product.
Why Acid Is Needed
The –OH group of an alcohol is a poor leaving group. In acidic conditions, it is protonated to form H₂O, a much better leaving group. This allows the alcohol molecule to undergo elimination more readily.
Leaving group: An atom or group that departs with an electron pair during a reaction.
After protonation, the alcohol becomes more reactive toward dehydration, enabling efficient alkene formation under heat.
Formation of the Alkene
Although the specification states that the mechanism is not required, it is still useful to understand the key conceptual steps that explain typical outcomes.
Key Transformational Steps (Conceptual Overview)
Protonation of the alcohol to convert –OH into H₂O.
Loss of water to form a reactive intermediate (carbocation or similar, depending on alcohol class).
Formation of the carbon–carbon double bond by removal of a proton from a neighbouring carbon.
One important consequence is that multiple alkenes can form if the alcohol allows different positions for double-bond formation. For instance, dehydration of butan-2-ol can yield but-1-ene or but-2-ene. Product distribution depends on the stability of the alkene formed.
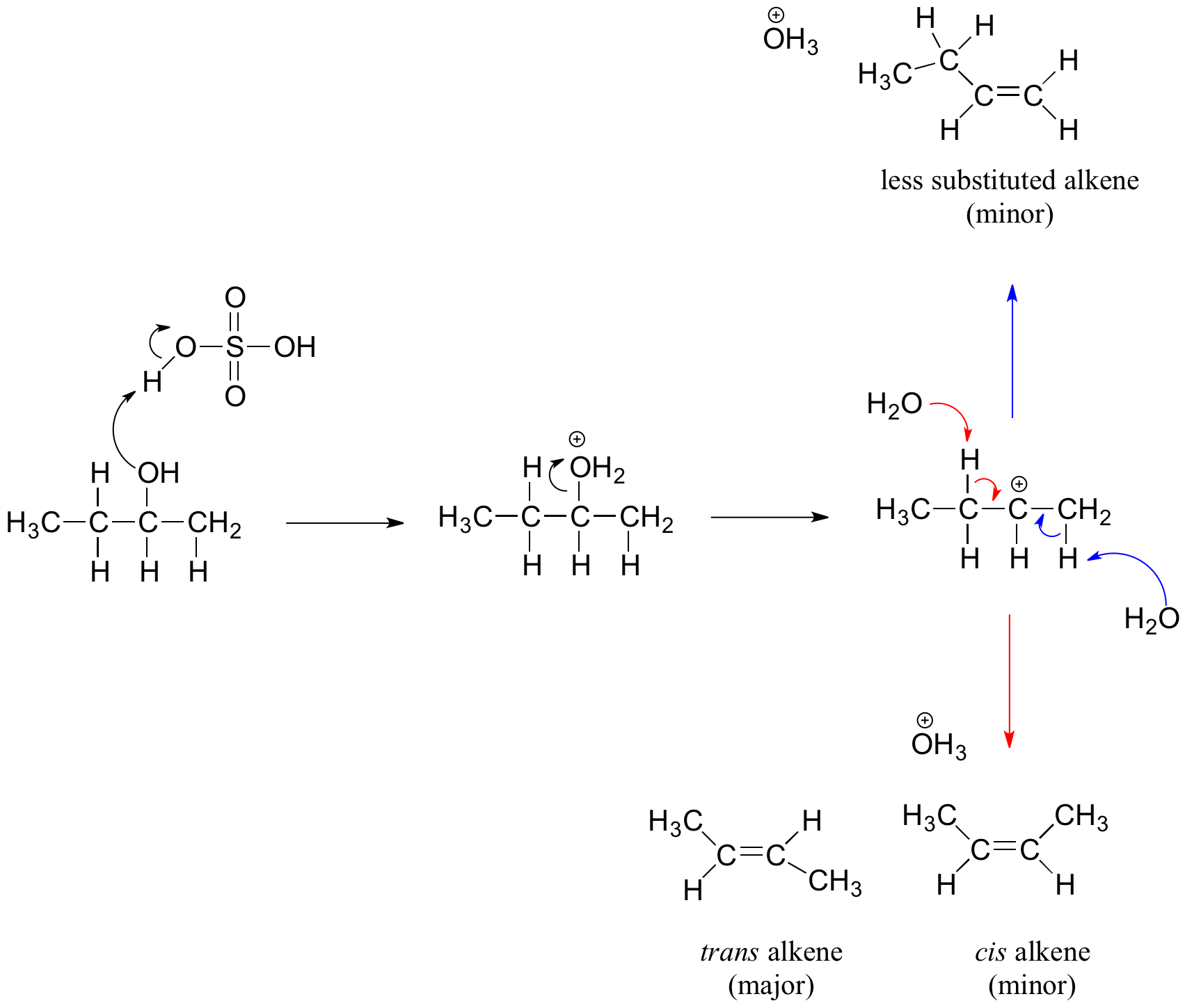
The diagram shows dehydration of a secondary alcohol forming several possible alkenes, with the more substituted trans‑2‑butene identified as the major product, reinforcing Zaitsev’s rule. Source
Regioselectivity and Product Distribution
Dehydration often follows Zaitsev’s rule, where the major product is typically the more substituted alkene, as these are more thermodynamically stable.
Zaitsev’s rule: In elimination reactions, the most substituted alkene tends to form preferentially.
More substituted alkenes have a lower overall energy due to hyperconjugation and inductive stabilisation. This explains the observed selectivity when more than one alkene is possible.
A short sentence ensures smooth flow before any further structured information is introduced.
Factors Affecting Product Distribution
Stability of possible alkenes (more substituted = more stable)
Carbocation stability (if relevant to the alcohol type)
Reaction temperature (higher temperatures can shift equilibria)
Acid strength and concentration
Dehydration of Different Classes of Alcohols
The reactivity of alcohols towards dehydration varies according to whether they are primary, secondary, or tertiary, due to differences in intermediate stability.
Primary Alcohols
Harder to dehydrate because intermediates formed are less stable.
Higher temperatures may be required.
Secondary Alcohols
Dehydrate more readily than primary alcohols.
Often give mixtures of alkenes that follow Zaitsev’s rule.
Tertiary Alcohols
Easiest to dehydrate due to stability of intermediates.
Usually form a single major alkene product.
Industrial and Laboratory Importance
Dehydration is an essential transformation for both industrial and laboratory syntheses. Alkenes produced in this way serve as:
Precursors for polymer manufacture (e.g., poly(ethene), poly(propene))
Starting materials for further functional group transformations
Intermediates in creating more complex organic molecules
In laboratory contexts, dehydration provides a straightforward method for converting accessible alcohols into more reactive unsaturated compounds that undergo addition reactions.
Practical Considerations for OCR Students
When learning dehydration as part of the OCR A-Level Chemistry course, focus on:
Recognising the required reagents: concentrated H₃PO₄ or H₂SO₄.
Understanding that water is eliminated to form an alkene.
Remembering that mechanisms are not examined for this subsubtopic.
Predicting possible alkenes and identifying the major product using Zaitsev’s rule.
These points align with the specification requirement to describe the process without needing mechanistic detail but with clear understanding of reagents, conditions and outcomes.
FAQ
The temperature depends largely on how easily the alcohol can lose water. Tertiary alcohols dehydrate at lower temperatures because they form stabilised intermediates more readily.
Primary alcohols require higher temperatures as the formation of their intermediates is less favourable.
Stronger acids and higher concentrations can also reduce the temperature needed for effective dehydration.
Rearrangements occur when the reaction briefly forms a carbocation that can shift to a more stable position before the alkene is generated.
This typically happens with secondary and tertiary alcohols, where hydride shifts or methyl shifts give a more stable carbocation.
The rearranged carbocation then leads to an alkene different from the one expected from the original alcohol structure.
Both concentrated phosphoric and sulfuric acids can dehydrate alcohols, but their properties influence side reactions.
Sulfuric acid is more oxidising and may promote unwanted oxidation or polymerisation.
Phosphoric acid is milder, offering cleaner dehydration, especially in laboratory synthesis where purity is important.
Distillation removes the alkene as it forms, shifting the equilibrium toward further product formation.
This prevents the reverse reaction, in which water adds back across the alkene to regenerate the alcohol.
It is particularly beneficial when producing volatile alkenes that can be separated easily from the reaction mixture.
Dehydration involves concentrated acids and high temperatures, so appropriate protective equipment is essential.
Key precautions include:
Wearing goggles, gloves and a lab coat.
Ensuring good ventilation due to flammable alkene vapours.
Using heat sources carefully to avoid overheating or pressure build-up in apparatus.
Practice Questions
Ethanol is dehydrated using concentrated phosphoric acid.
State the type of reaction taking place and name the organic product formed.
(2 marks)
1 mark: Correctly identifies the reaction as dehydration or elimination.
1 mark: Names the product as ethene.
Butan-2-ol is heated with concentrated sulfuric acid to form a mixture of alkenes.
Explain why more than one alkene is formed, and why one of these alkenes is produced in a greater amount than the others.
In your answer, refer to the possible structures of the alkenes formed and the factors influencing their relative proportions.
(5 marks)
Award marks for any of the following relevant points:
1 mark: Recognises that removal of water can occur in more than one position, leading to different alkenes.
1 mark: Identifies that butan-2-ol forms but-1-ene and but-2-ene.
1 mark: States that alkene stability varies depending on substitution.
1 mark: Explains that the more substituted alkene (but-2-ene) is more stable.
1 mark: Links higher stability to increased yield of but-2-ene (reference to Zaitsev’s rule acceptable).

