OCR Specification focus:
‘Determine specific heat capacity for metals or liquids using an electrical heating experiment.’
In this subtopic, students explore how to measure the specific heat capacity of substances such as metals and liquids using electrical heating. The method links measurable electrical quantities to the thermal energy transferred, forming a vital experimental foundation for understanding energy changes and thermal behaviour in materials.
Understanding Specific Heat Capacity
Specific heat capacity (c) is a property that defines how much energy is required to raise the temperature of a unit mass of a substance by one degree Celsius (°C) or one kelvin (K). It provides an indication of how effectively a material can store thermal energy.
Specific Heat Capacity: The energy required to raise the temperature of 1 kg of a substance by 1 K (or 1 °C).
The specific heat capacity varies between substances, depending on molecular structure and bonding. Metals, for example, tend to have lower values because their delocalised electrons transfer energy efficiently, whereas liquids like water have higher values due to strong intermolecular forces.
Principle of the Electrical Method
The electrical method for determining specific heat capacity uses the principle of electrical energy conversion. Electrical energy supplied by a heater (or resistor) is converted into thermal energy, which increases the internal energy of the substance and hence its temperature. By measuring the relevant quantities, we can relate electrical energy input to temperature change.
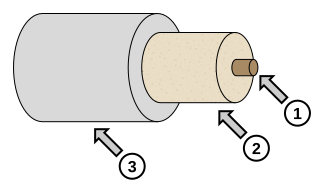
Labelled cross-section of a tubular electric heater showing its internal components. These parts enable efficient Joule heating of the sample. This additional detail illustrates how electrical energy is converted to heat in the experiment. Source.
EQUATION
—-----------------------------------------------------------------
Energy Transferred (ΔE) = Current (I) × Potential Difference (V) × Time (t)
I = current through the heater (A)
V = potential difference across the heater (V)
t = heating time (s)
—-----------------------------------------------------------------
Combining this with the general relationship for heating gives:
EQUATION
—-----------------------------------------------------------------
Specific Heat Capacity Relationship (ΔE = mcΔT)
ΔE = energy supplied (J)
m = mass of the substance (kg)
c = specific heat capacity (J kg⁻¹ K⁻¹)
ΔT = temperature rise (K or °C)
—-----------------------------------------------------------------
From these, the value of c can be determined as:
EQUATION
—-----------------------------------------------------------------
c = (I V t) / (m ΔT)
—-----------------------------------------------------------------
Between these two energy relationships, the electrical and thermal aspects are directly linked, providing a reliable way to calculate the specific heat capacity experimentally.
Apparatus and Key Components
A typical setup for this experiment includes:
A solid sample or liquid container whose specific heat capacity is to be measured.
An electric heater (usually a resistive element) inserted into or in contact with the sample.

A 500 W immersion heater used in laboratory experiments for resistive heating. In specific heat capacity investigations, this heater is placed in a liquid or metal block while current and voltage are monitored. Source.
A power supply to deliver a constant current and voltage.
A digital ammeter and voltmeter to monitor electrical quantities
A thermometer or thermocouple to measure temperature changes accurately.
A stopwatch or timer to record heating duration.
Insulation to reduce heat losses to the surroundings.
All components work together to allow measurement of the energy input and corresponding temperature rise.
Procedure Overview
Although later subtopics address techniques in greater depth, the overview of the electrical method can be summarised as follows:
Measure the mass of the sample accurately using a balance.
Assemble the circuit, connecting the power supply, ammeter, voltmeter, and heater.
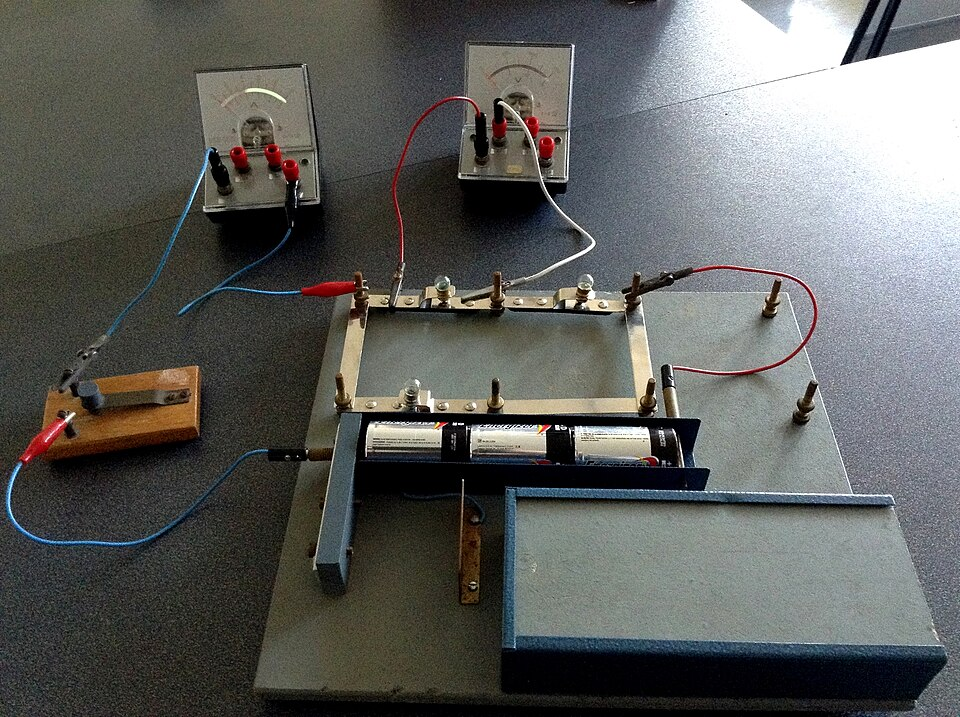
A demonstration circuit with an ammeter in series and a voltmeter in parallel to the heater. This arrangement allows measurement of current and potential difference during the heating process. Source.
Insert the thermometer or temperature probe into the sample to monitor temperature changes.
Switch on the power, allowing the heater to supply energy for a measured time period.
Record the voltage (V), current (I), and heating time (t) during this interval.
Measure the temperature increase (ΔT) of the sample once heating stops and equilibrium is reached.
Calculate the specific heat capacity using the equation c = (I V t)/(m ΔT)
This method is suitable for both metal blocks (where the heater fits into drilled holes) and liquids (where the heater and thermometer are immersed in a beaker or calorimeter).
Ensuring Accuracy and Minimising Errors
To obtain reliable results, several precautions must be taken:
Reduce heat losses by insulating the sample and surrounding the setup with lagging materials such as polystyrene.
Ensure good thermal contact between the heater, thermometer, and the sample for uniform temperature distribution.
Stir liquids gently to prevent temperature gradients.
Use calibrated instruments for current, voltage, and temperature measurements.
Avoid temperature overshoot, as latent heat or phase changes would invalidate the simple heating assumption.
Record all quantities carefully to minimise systematic and random errors.
Heat losses to the environment are the most significant source of error, as they cause the calculated specific heat capacity to appear larger than the true value.
Theoretical Considerations
The electrical method assumes that all electrical energy supplied to the heater is transferred to the substance as thermal energy. In reality, part of this energy is lost to:
The surrounding air via convection and radiation.
The apparatus, such as the container or heater itself.
The thermometer or probe, which also absorbs some heat.
These effects can be mitigated through insulation and corrections for the thermal capacity of the apparatus, ensuring that results better represent the true specific heat capacity of the material.
Experimental Analysis
When analysing results:
The gradient of a temperature–time graph can indicate the rate of heating.
Plotting ΔE versus ΔT can yield a straight line, with the gradient = m c.
Repeated measurements improve precision and allow averaging of results.
These techniques reinforce the relationship between energy input and temperature rise, central to understanding energy storage in matter.
Applications and Relevance
This experiment is essential because it illustrates key thermal physics principles that underpin many real-world applications:
Engineering and materials science use specific heat data to design components exposed to heat.
Climate and environmental physics rely on understanding water’s high specific heat capacity for modelling temperature stability.
Electronics and thermodynamics involve managing heat transfer efficiently using known material properties.
Thus, mastering this experimental approach provides both conceptual understanding and practical laboratory skills, preparing students for advanced study and scientific applications.
FAQ
The primary sources of heat loss include:
Conduction through the apparatus such as the heater leads or thermometer stem.
Convection and radiation to the surrounding air.
Heating of the container or mounting structure rather than just the sample.
These losses mean that not all the electrical energy calculated from IVt is transferred to the sample, causing an overestimation of the specific heat capacity since ΔE appears larger than the true thermal energy absorbed by the material.
Metal blocks are easier to insulate and handle. They provide:
A solid shape allowing good contact between heater and thermometer.
Minimal evaporation or spillage compared to liquids.
Faster attainment of uniform temperature due to higher thermal conductivity.
Liquids, by contrast, require more careful insulation and stirring to achieve uniform heating and prevent convection currents from skewing the temperature readings.
To reduce uneven heating:
Ensure the heater is centrally embedded within the metal block.
Use a thermocouple or digital temperature probe close to the heater rather than a surface thermometer.
For liquids, stir gently but continuously during heating.
These steps help minimise temperature gradients, ensuring that the measured ΔT better represents the entire sample, leading to more accurate specific heat capacity results.
The calculation of electrical energy assumes that both current (I) and voltage (V) remain steady throughout the heating period.
If either fluctuates:
The product IVt no longer accurately represents total energy supplied.
Heating may become inconsistent, causing errors in ΔT measurement.
Using a regulated power supply or recording values at frequent intervals and calculating an average can greatly improve reliability in determining specific heat capacity.
For irregular samples, direct heating may be impractical. Instead:
Immerse the metal in a known mass of water within a calorimeter.
Use the heater to warm both the water and metal simultaneously.
Measure ΔT of the water and assume thermal equilibrium between the two.
Apply the principle of conservation of energy, equating electrical energy supplied to the combined heat gain of water and metal.
By rearranging, the metal’s specific heat capacity can be deduced indirectly from measurable quantities.
Practice Questions
Question 1 (2 marks)
A student heats a 1.00 kg block of aluminium using an electrical heater connected to a 12.0 V power supply drawing a steady current of 3.0 A for 200 s.
Explain how the student could use these quantities to determine the specific heat capacity of aluminium.
Mark Scheme:
1 mark: Recognises that electrical energy supplied equals heat energy gained by the block, using ΔE = IVt.
1 mark: States or implies that c = (IVt)/(mΔT) and that ΔT is obtained by measuring the temperature change of the block.
Question 2 (5 marks)
Describe how an experiment using an electrical heater can be set up to determine the specific heat capacity of a metal block. In your answer, include the measurements required, the equations used, and how accuracy can be improved.
Mark Scheme:
1 mark: States that a metal block is fitted with a heater and thermometer (or thermocouple) to measure temperature change.
1 mark: Mentions that current (I), potential difference (V), and heating time (t) are measured to find energy supplied (ΔE = IVt).
1 mark: States that the mass (m) of the block is measured and that the temperature change (ΔT) is recorded.
1 mark: States that the specific heat capacity is calculated using c = (IVt)/(mΔT).
1 mark: Describes at least one method of improving accuracy, such as using insulation to reduce heat loss, ensuring good thermal contact, or applying a correction for heat absorbed by the apparatus.

