OCR Specification focus:
‘Explain how gamma camera images are used in diagnosis with examples.’
Gamma cameras provide clinicians with functional images of internal organs by detecting gamma radiation from administered radioactive tracers, enabling diagnosis of disease through visualising physiological processes.
How Gamma Camera Images Support Diagnosis
A gamma camera produces an image showing the distribution of a radioactive medical tracer within the patient’s body. These images are functional rather than anatomical, giving clinicians insight into how organs work rather than how they look structurally. Because the distribution of the tracer reflects metabolic activity, blood flow, or cellular function, abnormalities appear as regions of unusually high or low activity, allowing clinicians to detect and assess disease.
The tracer used in gamma-camera imaging typically emits gamma photons, which are detected after passing through the camera’s components. Abnormal tracer uptake provides diagnostic clues that complement or surpass findings from conventional structural imaging.
Principles Behind Diagnostic Interpretation
Diagnostic use of gamma-camera images relies on recognising patterns of hot spots (regions of higher-than-normal radioactivity) and cold spots (regions of lower-than-normal radioactivity). These patterns are linked directly to changes in physiological function.
Hot spot: An area on a gamma-camera image where tracer concentration is abnormally high, appearing bright due to increased gamma photon emission.
A normal sentence must follow, ensuring the block above stands alone before moving on to new definitions.
In contrast, cold spots appear when a tissue or organ fails to take up the tracer as expected. Both types of features provide valuable evidence for diagnosis, depending on the clinical context and tracer used.
Cold spot: A region on a gamma-camera image where tracer uptake is abnormally low, indicating reduced physiological activity.
Gamma-camera images are therefore assessed for position, size, edge clarity, and intensity of these areas. When interpreting scans, radiologists also consider patient history, tracer pharmacokinetics, and expected organ behaviour.
Clinical Applications in Diagnosis
Bone Scintigraphy
One of the most common diagnostic applications is bone scintigraphy, used to assess bone health and detect abnormalities.
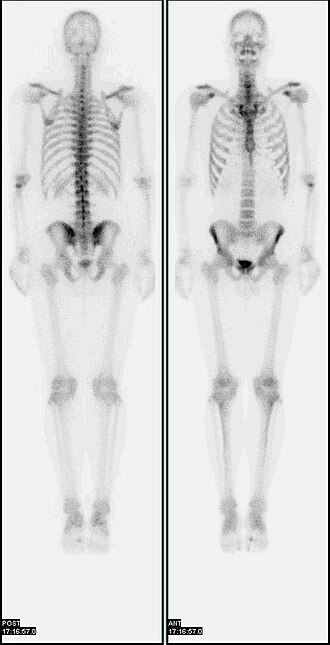
A whole-body bone scintigraphy image obtained with a gamma camera, showing tracer uptake throughout the skeleton. Areas of increased uptake appear as brighter “hot” regions, which may correspond to sites of high bone turnover such as metastases, fractures, or infection. This image includes overall skeletal detail beyond the OCR specification but is used purely to illustrate how clinicians look for abnormal patterns of tracer distribution. Source.
Key uses include:
Identifying bone metastases, where cancer spreads to bone, producing distinct hot spots due to elevated metabolic activity from tumour-induced bone changes.
Diagnosing fractures, especially stress fractures, which may be invisible on standard X-rays early on.
Detecting bone infections (osteomyelitis) through areas of high tracer uptake at infection sites.
Because tracer accumulates where bone turnover is increased, bone scintigraphy is highly sensitive, making it valuable for early detection.
Thyroid Function Assessment
Gamma-camera imaging plays a central role in assessing thyroid disorders, as the thyroid gland naturally concentrates certain tracers.
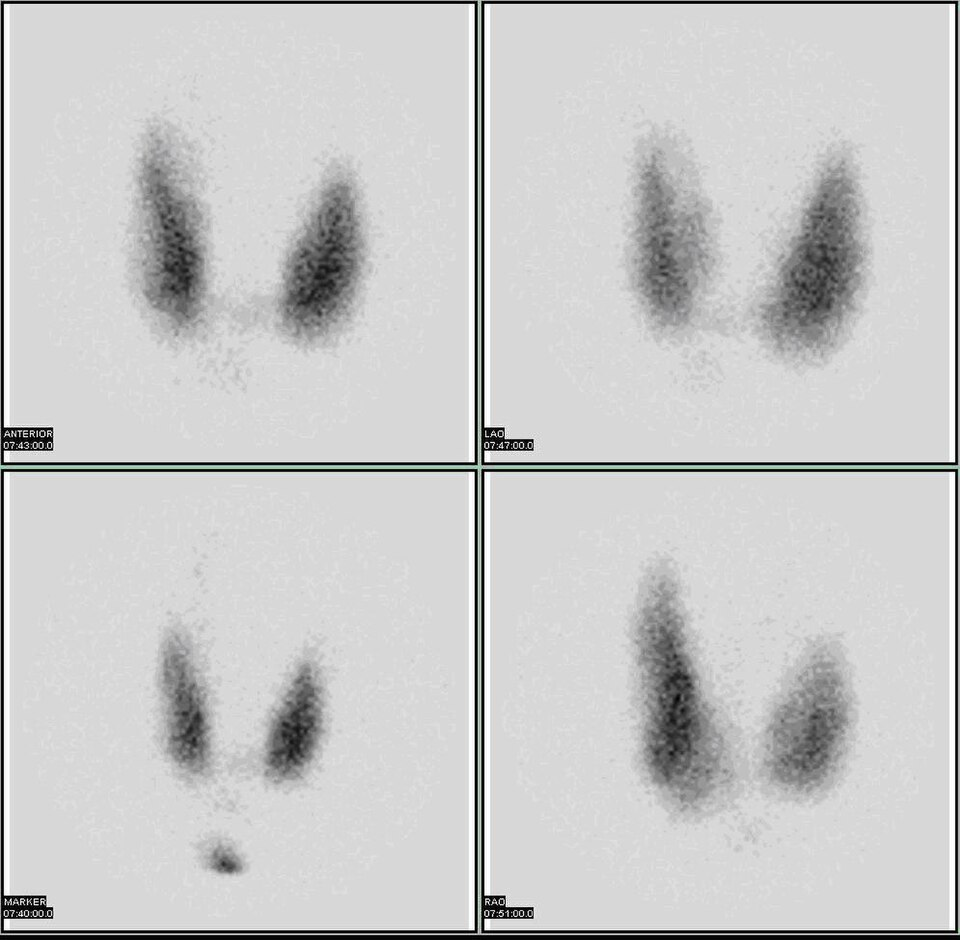
A planar gamma-camera thyroid scan using an iodine-123 tracer, showing the functional distribution of activity within the thyroid gland. Brighter areas represent higher tracer uptake (“hot” regions), whereas darker or absent areas correspond to reduced uptake (“cold” regions), which may indicate non-functioning nodules or other pathology. The image does not label specific diseases, so it remains closely aligned to the OCR requirement of understanding how patterns of tracer uptake support diagnosis. Source.
Diagnostic information includes:
Hot spots indicating hyperfunctioning nodules, which may correspond to benign overactive tissue.
Cold spots identifying non-functioning nodules, which can be benign or malignant and therefore require further investigation.
Overall gland activity patterns for diagnosing conditions such as Graves’ disease or Hashimoto’s thyroiditis.
Renal Imaging
The kidneys are routinely studied using gamma-camera imaging to evaluate both perfusion and excretory function.
Clinically useful diagnostic outputs include:
Differentiating obstructive from non-obstructive hydronephrosis by observing tracer flow through the renal pelvis.
Assessing renal perfusion, helping detect arterial narrowing or poor functional tissue.
Monitoring transplanted kidneys, as abnormal uptake can indicate rejection or impaired blood supply.
Cardiac Imaging
Gamma-camera imaging enables assessment of myocardial perfusion, especially in suspected coronary artery disease.
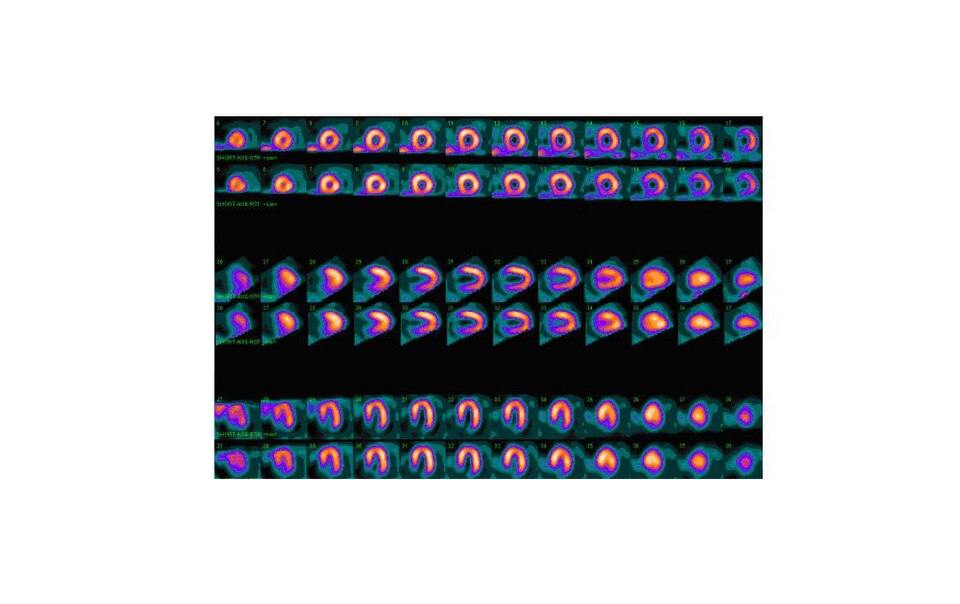
A nuclear medicine myocardial perfusion scan showing normal tracer uptake throughout the left ventricular myocardium, with no visible perfusion defects. The circular and semi-circular slices represent different tomographic views of the heart, each demonstrating uniform gamma-emitting tracer distribution. The presence of tomographic detail goes slightly beyond the OCR wording but remains highly relevant for visualising what “normal perfusion” looks like when interpreting gamma-camera cardiac images. Source.
Important diagnostic applications involve:
Identifying ischaemic regions that receive insufficient blood flow under exercise or pharmacological stress.
Distinguishing between reversible perfusion defects (suggesting treatable blockages) and fixed defects (indicating infarcted tissue).
Supporting risk stratification and treatment planning for cardiac patients.
Sentinel Lymph Node Identification
Gamma-camera imaging is widely used for sentinel lymph node mapping, particularly in cancers such as breast cancer or melanoma.
Key diagnostic benefits include:
Locating the first lymph node or nodes draining the tumour site.
Guiding surgeons to remove only the relevant nodes, reducing unnecessary tissue removal.
Detecting metastatic spread at an early stage.
How Clinicians Interpret Gamma Camera Data
Recognising Patterns
Clinicians examine:
The symmetry of tracer uptake across paired organs.
The shape and definition of abnormal regions.
The time-dependent behaviour of the tracer in dynamic studies.
The relative intensities of regions to determine over- or under-activity.
Correlating with Physiology
Interpretation always considers the biological behaviour of the tracer. For instance, a tracer localised by bone turnover provides metabolic information, whereas a tracer filtered by the kidneys shows renal perfusion and excretion.
Integration into Clinical Decision-Making
Gamma-camera findings are combined with:
Patient symptoms and history
Blood test results
Other imaging modalities (e.g., CT or ultrasound)
Clinical examination
This multi-modal approach ensures that functional information from gamma-camera imaging contributes effectively to forming diagnoses, guiding treatment, and monitoring disease progression.
FAQ
Artefacts often arise from patient movement, contamination from spilled tracer, or equipment-related issues such as photomultiplier imbalance.
Clinicians check for symmetry, repeatability across different views, and whether the abnormal region aligns with anatomical expectations.
They may also compare static and dynamic images, or refer to prior scans to determine whether a feature is reproducible.
Common artefacts include:
Linear streaks caused by detector faults
Localised bright spots from tracer residue on the skin
Blurred areas from breathing or movement during acquisition
Careful positioning and quality-control procedures help minimise these issues.
Gamma cameras detect gamma photons that originate from within the body, but these photons cannot be focused using lenses or mirrors.
The collimator selects photons travelling in specific directions, which significantly reduces the number of detected photons. This improves spatial accuracy but decreases resolution.
Additional factors limiting resolution include:
The intrinsic resolution of the scintillator and photomultiplier array
Photon scatter within the patient
The need to limit acquisition time to avoid excessive dose
Despite lower resolution, the functional detail provided is extremely valuable for diagnosis.
Different tracers localise in tissues based on biochemical properties, allowing clinicians to target specific physiological processes.
An unsuitable tracer may:
Show poor uptake in the organ of interest
Clear too quickly or too slowly for imaging
Produce gamma photons with energies incompatible with the camera’s design
Tracer choice also affects patient dose and image clarity. For example, tracers emitting photons around 140 keV are ideal for standard gamma cameras because they balance tissue penetration with detector efficiency.
Dynamic imaging records the movement of tracer through the body over time, rather than relying on a single static image.
This allows clinicians to observe:
The rate of tracer uptake and washout
Perfusion patterns in organs such as the kidneys or heart
Functional delays, blockages, or impaired transport mechanisms
Dynamic curves plotted from regions of interest enable quantitative assessments such as renal filtration rates or time-activity relationships, supporting more detailed physiological analysis.
Dose depends primarily on the tracer’s activity and physical properties.
Key contributors include:
Tracer half-life
Biological clearance rate
Photon energy emitted
Amount of activity administered
Other factors include imaging duration and whether additional views or dynamic sequences are required.
Modern protocols minimise dose by choosing short-lived tracers and using sensitive detectors that require lower administered activity.
Practice Questions
Question 1 (2 marks)
Explain what is meant by a hot spot on a gamma-camera image and state what a clinician might infer from its presence.
Question 1 (2 marks)
• Hot spot correctly defined as a region of abnormally high tracer uptake or increased gamma photon emission (1)
• Appropriate clinical interpretation, e.g. indicates increased metabolic activity, overactive tissue, inflammation, or possible tumour/metastasis (1)
Question 2 (5 marks)
Gamma-camera imaging is widely used to diagnose disorders in organs such as the thyroid and heart. Describe how gamma-camera images are formed and explain how clinicians use the patterns of tracer uptake to diagnose disease in these organs. Your answer should include reference to both hot and cold regions and at least one specific diagnostic application.
Question 2 (5 marks)
• Gamma photons emitted from a radioactive tracer inside the patient are detected by the gamma camera to produce an image of tracer distribution (1)
• Hot regions identified as areas of abnormally high tracer uptake; cold regions identified as areas of abnormally low uptake (1)
• Explanation that patterns of uptake indicate levels of physiological activity or function within the organ (1)
• Relevant diagnostic detail for the thyroid, e.g. hot nodule suggests overactive tissue; cold nodule may suggest non-functioning or potentially malignant tissue (1)
OR
• Relevant diagnostic detail for the heart, e.g. reduced uptake in myocardial perfusion imaging indicates ischaemia or infarction (1)
• Overall link to how clinicians interpret these patterns to make a diagnosis, referring explicitly to functional abnormalities rather than anatomical ones (1)

