OCR Specification focus:
‘Know the S.I. base quantities and their units: kg, m, s, A, K, mol.’
Understanding S.I. base quantities and units is fundamental in A-Level Physics. These form the foundation for all physical measurements, allowing scientists to describe, compare, and communicate results consistently worldwide.
The International System of Units (S.I.)
The Système International d’Unités (S.I.) is the globally accepted system for measuring physical quantities. It ensures standardisation across scientific disciplines and countries, allowing measurements to be reproducible, precise, and universally comparable. Every measurable physical quantity can be expressed in terms of base quantities and their corresponding S.I. base units.
The Concept of Base Quantities
Base quantities are the most fundamental measurable properties in physics. They cannot be defined in terms of other physical quantities. All other quantities—known as derived quantities—are constructed from these bases through multiplication, division, or powers.
Base Quantity: A fundamental physical property that cannot be expressed in terms of other quantities.
Each base quantity is associated with a specific S.I. base unit that provides a standard reference for measurement.
The Seven S.I. Base Quantities and Units
There are seven recognised S.I. base quantities. Each represents a fundamental aspect of physical measurement.
1. Length (metre, m)
The metre (m) is the S.I. base unit for length. It represents the spatial distance between two points.
Defined in terms of the speed of light: one metre is the distance light travels in 1/299,792,458 seconds in a vacuum.
A concise illustration of the metre (m) defined by fixing the speed of light in vacuum, c = 299 792 458 m s⁻¹. The diagram highlights that the metre derives directly from the second and the universal constant c. Extra detail beyond the OCR syllabus is minimal and confined to naming the constant. Source.
Used to measure displacement, wavelength, radius, and similar quantities.
2. Mass (kilogram, kg)
The kilogram (kg) measures the amount of matter in an object.
Since 2019, the kilogram is defined using the Planck constant (h), rather than a physical object.
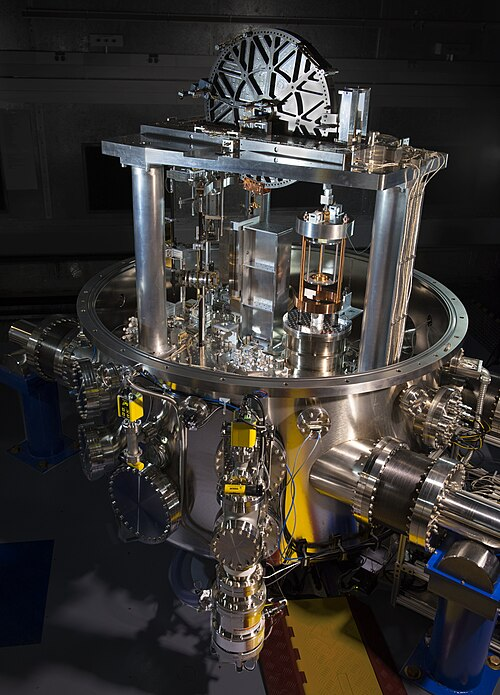
The NIST-4 Kibble balance, used to realise the kilogram (kg) from the fixed value of the Planck constant. It balances mechanical against electromagnetic power to link mass to fundamental constants. The engineering detail visible goes beyond the OCR syllabus but simply evidences the constant-based definition. Source.
This ensures the definition remains constant and universally reproducible.
Commonly used for measuring inertia and gravitational effects.
3. Time (second, s)
The second (s) is the base unit for time, representing the duration of an event or the interval between events.
Defined as the duration of 9,192,631,770 cycles of radiation corresponding to the transition between two hyperfine levels of the caesium-133 atom.
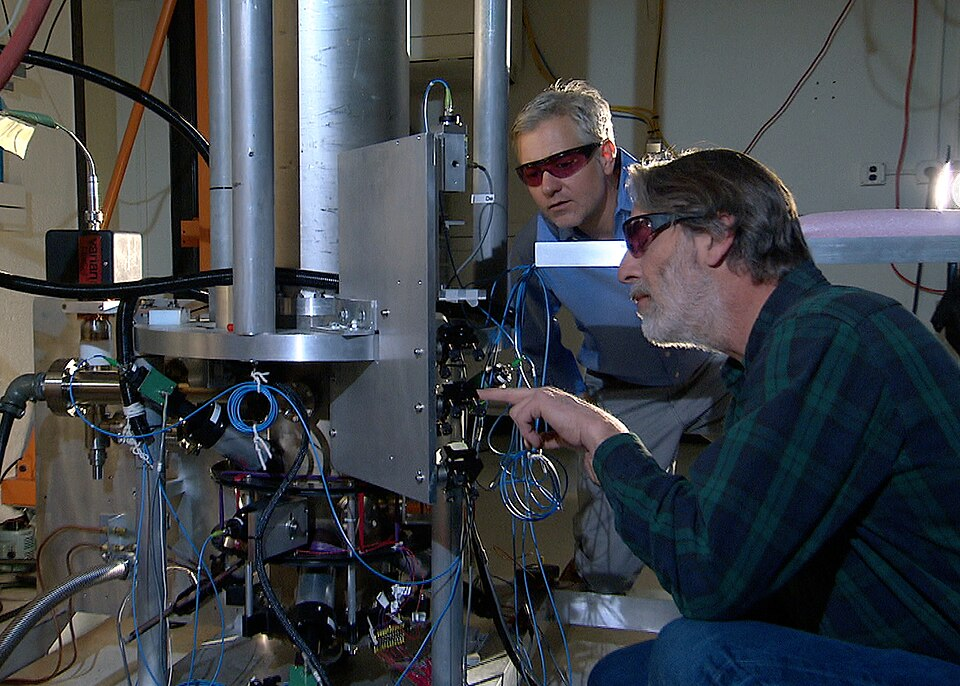
The NIST-F2 cesium fountain atomic clock, which realises the second (s) from the caesium-133 hyperfine transition at 9 192 631 770 Hz. The image shows the vacuum chamber and support structure used to launch and interrogate cold caesium atoms. Laboratory specifics visible exceed the syllabus but directly illustrate the current definition. Source.
Defined as the duration of 9,192,631,770 cycles of radiation corresponding to the transition between two hyperfine levels of the caesium-133 atom.
Central to measurements of velocity, frequency, and acceleration.
4. Electric Current (ampere, A)
The ampere (A) measures the rate of flow of electric charge through a conductor.
Electric Current: The rate at which electric charge passes through a point or conductor.
Defined in terms of the elementary charge (e), where one ampere corresponds to a flow of 1/(1.602176634 × 10⁻¹⁹) elementary charges per second.
Used extensively in electromagnetism and circuit theory.
5. Thermodynamic Temperature (kelvin, K)
The kelvin (K) quantifies temperature, specifically the average kinetic energy of particles in a system.
Defined relative to the Boltzmann constant (k), linking microscopic particle motion to macroscopic thermal energy.
Zero kelvin represents absolute zero, where molecular motion theoretically ceases.
6. Amount of Substance (mole, mol)
The mole (mol) expresses the number of entities (atoms, molecules, ions) in a substance.
Mole: The amount of substance containing exactly 6.02214076 × 10²³ specified entities (Avogadro’s number).
This quantity connects microscopic particle numbers to measurable quantities of matter in the laboratory.
Used widely in chemistry and particle physics.
7. Luminous Intensity (candela, cd)
The candela (cd) measures the perceived power of light emitted by a source in a specific direction.
Defined by fixing the value of the luminous efficacy of monochromatic radiation at a frequency of 540 × 10¹² hertz.
Although more relevant to photometry, it remains a base quantity because it defines light emission objectively.
Importance of S.I. Base Quantities
S.I. base quantities are vital because:
They provide a common language for all physical measurements.
They enable conversion and comparison between systems with accuracy.
They ensure equation consistency, allowing derived units to be checked through dimensional analysis.
They underpin all experimental and theoretical work in physics.
Without this standardisation, results from different experiments could not be accurately compared or reproduced.
Derived Units and Dimensional Consistency
From base units, we construct derived units—quantities that combine base dimensions. For instance:
Velocity is derived from length and time.
Force combines mass, length, and time through Newton’s second law.
EQUATION
—-----------------------------------------------------------------
Force (F) = mass × acceleration
F = m × a
m = mass (kg)
a = acceleration (m s⁻²)
—-----------------------------------------------------------------
This gives the derived unit for force as kg m s⁻², known as the newton (N).
Dimensional analysis confirms that equations like this are homogeneous—that is, all terms have equivalent dimensions.
The Evolution of S.I. Definitions
Recent advances have replaced artefact-based definitions with fundamental constants of nature, improving accuracy and long-term stability.
The metre relies on the speed of light.
The kilogram depends on the Planck constant (h).
The ampere references the elementary charge (e).
The kelvin is tied to the Boltzmann constant (k).
The mole is linked to Avogadro’s constant (Nₐ).
This approach ensures that the S.I. system is independent of physical artefacts, such as the former International Prototype Kilogram, which could change over time.
Conventions in S.I. Notation
When recording measurements:
Always use lowercase letters for unit symbols (e.g., m, s, mol) except for those derived from proper names (e.g., A for ampere, K for kelvin).
Unit symbols are not pluralised (e.g., 5 kg, not 5 kgs).
There should be a space between the value and unit (e.g., 2.5 m).
Prefixes such as milli-, kilo-, or mega- may be applied, but the base units remain unchanged in meaning.
Summary of Base Quantities and Units
Length — metre (m)
Mass — kilogram (kg)
Time — second (s)
Electric current — ampere (A)
Thermodynamic temperature — kelvin (K)
Amount of substance — mole (mol)
Luminous intensity — candela (cd)
These form the cornerstones of the S.I. system, enabling the coherent and reliable expression of all physical phenomena.
FAQ
The seven S.I. base quantities were chosen because they form a complete and independent set of physical quantities from which all others can be derived.
Each represents a distinct aspect of the physical world — for example, mass and electric current describe different phenomena that cannot be expressed in terms of one another.
Other measurable properties, like force or pressure, can be built from these base quantities, meaning no additional fundamental quantities are required.
Revisions are made by the General Conference on Weights and Measures (CGPM), based on recommendations from international scientific bodies like the International Committee for Weights and Measures (CIPM).
Changes occur only when measurement science advances enough to define units using fundamental physical constants rather than artefacts or unstable references.
For example, in 2019 all base units were redefined in terms of constants such as h, c, and k, ensuring long-term accuracy and stability.
Originally, the kilogram was defined as the mass of one cubic decimetre of water. The base unit of mass thus became the kilogram, not the gram, which already included the “kilo-” prefix.
This historical choice persisted to maintain continuity, even though it breaks the usual rule of prefix-free base unit names.
Other mass units, such as milligram or tonne, are derived from the kilogram rather than directly from the gram.
Each unit is realised using an approved method that links it to fixed physical constants. For example:
The metre is realised using laser interferometry and the known speed of light.
The kilogram is realised via a Kibble balance, measuring the Planck constant.
The second is realised through atomic clocks based on caesium-133 transitions.
These realisations are coordinated by national measurement institutes (like NPL in the UK) to ensure worldwide uniformity.
It’s theoretically possible but highly unlikely. Any new base quantity would need to describe a fundamentally independent aspect of nature not expressible through existing base quantities.
So far, all measurable quantities in physics can be derived from the existing seven, including those in emerging fields like quantum or particle physics.
Adding a new base quantity would only be justified if new physics revealed a distinct, irreducible dimension requiring its own unit.
Practice Questions
Question 1 (2 marks)
State two of the S.I. base quantities and give the corresponding S.I. base units.
Mark Scheme
1 mark for correctly identifying each base quantity (max 2)
1 mark for correctly giving its corresponding S.I. base unit (must match the quantity)
Acceptable answers (any two of the following pairs):
Length – metre (m)
Mass – kilogram (kg)
Time – second (s)
Electric current – ampere (A)
Thermodynamic temperature – kelvin (K)
Amount of substance – mole (mol)
Luminous intensity – candela (cd)
Question 2 (5 marks)
Explain why the S.I. base quantities and units are important in physics. Include in your answer how they contribute to the accuracy and consistency of scientific measurement and how modern definitions based on physical constants improve the reliability of these units.
Mark Scheme
1 mark for stating that S.I. base quantities provide a standard or universal reference system used globally in physics.
1 mark for explaining that they allow consistent and comparable measurements between different experiments, scientists, and countries.
1 mark for noting that derived quantities can be expressed in terms of base quantities, ensuring equation homogeneity and dimensional consistency.
1 mark for describing that modern S.I. units are now defined by fixed values of fundamental physical constants (e.g., speed of light, Planck constant, Boltzmann constant).
1 mark for explaining that using fundamental constants removes reliance on physical artefacts, increasing precision, stability, and reproducibility of measurements over time.

