AP Syllabus focus:
‘Describe how nitrogen, phosphorus, and sulfur contribute to the structure of proteins, phospholipids, and nucleic acids.’
Nitrogen, phosphorus, and sulfur are “functional” elements: they add key chemical groups that control macromolecular shape, charge, bonding, and reactivity. Their positions within monomers strongly influence polymer architecture and biological interactions.
Why These Elements Matter for Macromolecular Structure
Common Structural Themes
Electronegativity and bonding: N, P, and S form polar covalent bonds that create partial charges, affecting folding and intermolecular attraction.
Charge: phosphate-containing groups are often negatively charged, while many nitrogen-containing groups can become positively charged depending on pH.
Cross-linking potential: sulfur can create covalent links that lock a protein into a stable conformation.
Nitrogen (N) in Proteins, Nucleic Acids, and Phospholipids
Proteins: N Enables Peptide Formation and Side-Chain Chemistry
Amino acids contain an amine group (–NH₂/–NH₃⁺), which supplies nitrogen to the polypeptide backbone and supports predictable geometry along the chain.
Peptide bond: A covalent bond linking the carboxyl carbon of one amino acid to the amino nitrogen of another, forming a continuous polypeptide backbone.
Because the backbone repeats N-containing amide groups, it presents consistent hydrogen-bond donors/acceptors that help establish stable local conformations. Nitrogen is also present in some R groups, altering polarity and charge.
Basic side chains (e.g., lysine, arginine, histidine) contain nitrogen and can become positively charged, contributing to ionic interactions within proteins and with other molecules.
Nitrogen-rich regions often interact strongly with negatively charged groups, especially phosphates.
Nucleic Acids: N Builds Nitrogenous Bases and Controls Pairing
In DNA and RNA, nitrogen is concentrated in the nitrogenous bases (purines and pyrimidines).
This diagram shows complementary base pairing in DNA, highlighting that adenine–thymine form two hydrogen bonds while cytosine–guanine form three. It visually connects nitrogen-containing functional groups in the bases to the specific hydrogen-bonding patterns that stabilize the double helix. Source
The arrangement of nitrogens in ring structures determines:
Hydrogen-bonding patterns that support complementary base pairing
Molecular recognition by enzymes that “read” grooves in the helix via base-edge chemistry
Even small changes in nitrogen placement can alter hydrogen-bond donor/acceptor patterns and thus the stability and fidelity of base pairing.
Phospholipids: N Can Shape Head-Group Identity
Many phospholipids include nitrogen-containing head groups (for example, choline or ethanolamine). These nitrogens can:
Contribute a permanent or pH-dependent positive character
Help create a zwitterion (both positive and negative charges), influencing packing and interactions at the membrane surface
Phosphorus (P) in Nucleic Acids and Phospholipids
Nucleic Acids: P Forms the Sugar–Phosphate Backbone
Phosphorus is present in phosphate groups that connect sugars, creating the repeating sugar–phosphate backbone. This backbone is crucial structurally because it:
Provides strong covalent continuity through phosphodiester linkages
Confers an overall negative charge, promoting interactions with water and with positively charged proteins and ions
Positions bases outward for pairing while keeping the backbone as a supportive scaffold
Phosphodiester linkage: The covalent bond in nucleic acids where a phosphate connects the 3′ carbon of one sugar to the 5′ carbon of the next sugar, forming the backbone.
That repeating phosphate geometry helps maintain consistent spacing and contributes to the helix’s uniform diameter and stability in aqueous environments.
Phospholipids: P Defines Amphipathic Architecture
In phospholipids, a phosphate-containing region forms the polar head, which:
Interacts with water via charge and polarity
Anchors head-group diversity that changes membrane surface properties (hydration, charge distribution, binding sites)
The phosphate’s negative charge is a major reason membranes have distinctive electrostatic surfaces that influence protein association and lipid organization.
Sulfur (S) in Protein Structure
Sulfur-Containing Amino Acids and Covalent Stabilisation
Sulfur occurs mainly in the amino acids cysteine and methionine. Its structural importance is clearest in cysteine, which can form disulfide bonds.
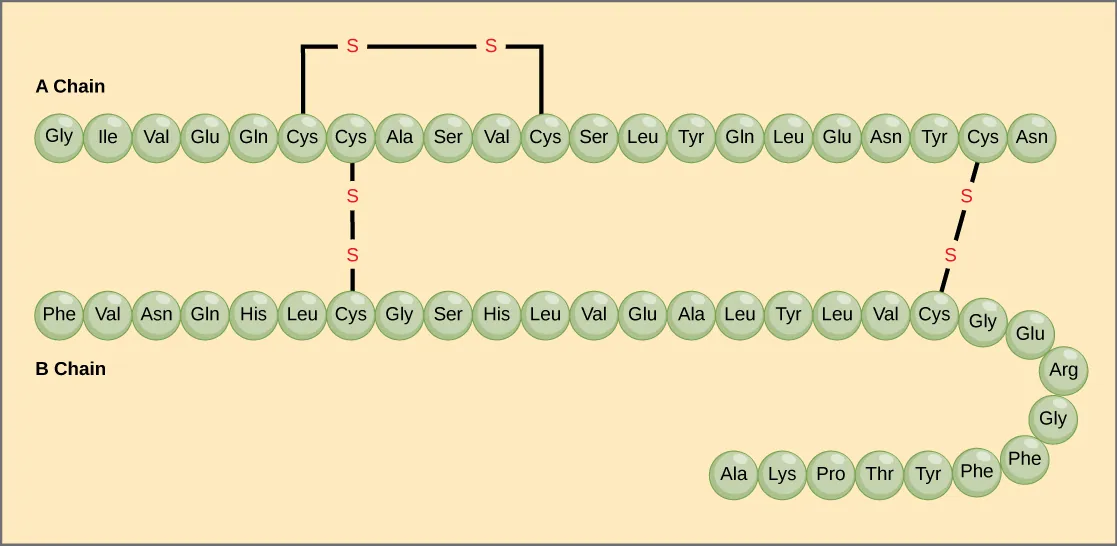
This figure uses insulin as an example to illustrate how disulfide (S–S) bonds form when two cysteine side chains oxidize and covalently link. The cross-links shown between and within polypeptide chains demonstrate how sulfur chemistry can constrain flexibility and stabilize a protein’s three-dimensional shape. Source
Disulfide bridges (S–S) create covalent cross-links within a polypeptide or between polypeptides
These cross-links reduce flexibility and help stabilise a protein’s folded shape, especially in oxidising environments such as extracellular spaces
Sulfur can also influence local polarity and packing because it is larger and more polarizable than oxygen, subtly affecting how side chains fit together in a folded protein.
FAQ
Phosphate has multiple oxygen atoms that stabilise negative charge by resonance.
At physiological pH, one or more protons dissociate, leaving a persistent negative charge on the group.
Disulfide bonds form more readily in oxidising conditions and are less stable in reducing conditions.
This is why many secreted proteins are disulfide-rich, while cytosolic proteins often are not.
No. Some head groups include nitrogen (e.g., choline, ethanolamine), while others do not (e.g., inositol).
Nitrogen-containing head groups often change surface charge balance and hydration.
Nitrogen placement controls hydrogen-bond donor/acceptor patterns and base stacking geometry.
Rare shifts in base nitrogen positions can alter pairing behaviour and local helix stability.
A disulfide bond is covalent, so it resists thermal motion more than noncovalent interactions.
It can “lock in” long-range connections, constraining folding pathways and final conformations.
Practice Questions
Explain one way phosphorus contributes to the structure of nucleic acids. (2 marks)
Phosphate groups form part of the sugar–phosphate backbone (1)
Phosphodiester bonds link nucleotides / create a repeating, negatively charged backbone that supports the strand (1)
Describe how nitrogen and sulfur contribute to protein structure. (5 marks)
Nitrogen is present in the amino group of amino acids / in the polypeptide backbone (1)
Peptide bonds form involving amino nitrogen, creating a continuous chain (1)
Nitrogen in some R groups can be positively charged, allowing ionic interactions that affect folding (1)
Sulfur is present in cysteine (1)
Disulfide bridges form between cysteines, stabilising tertiary/quaternary structure (1)

